Extended-Release Carbidopa/Levodopa Improves Motor Fluctuations in Parkinson Disease
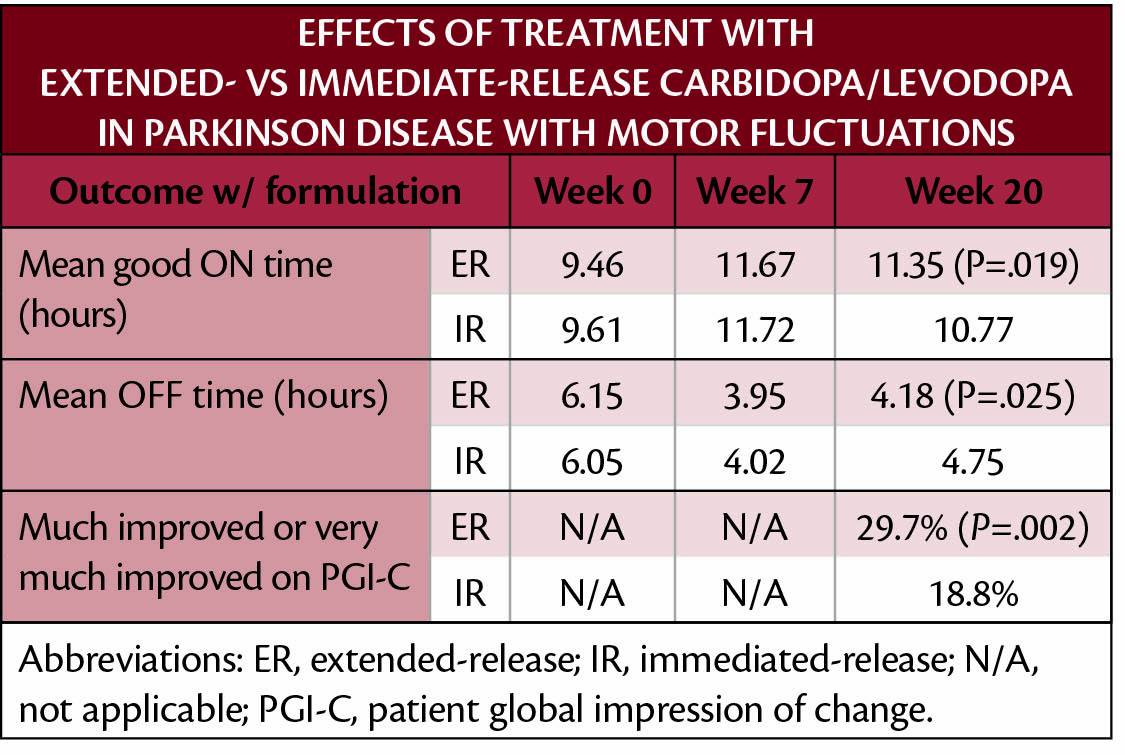
Data from a clinical trial (NCT03670953) show that a new formulation of extended-release carbidopa (CD)/levodopa (LD) (IPX-203; Amneal, Bridgewater, NJ) improved motor fluctuations of Parkinson disease (PD) compared with an immediate-release formulation. Good ON time was increased by 70% per dose with extended- vs immediate-release CD/LD.
Participants with Parkinson disease (PD) with motor fluctuations had a 3 week adjustment of their immediate-release CD/LD and then switched to equivalent doses of extended-release CD/LD for 4 weeks. After this 7-week baseline period, participants were randomly assigned to receive extended- or immediate-release formulations. As shown in the Table, increases in good ON time and decreases in OFF time were statistically significant with the extended- vs immediate-release formulations. Participants treated with extended-release CD/LD were also more likely to report subjective improvement on the Patient Global Impression of Change (PGI-C) scale.
“The topline data indicates that IPX-203 has the potential to offer patients superior good ON time with reduced dosing frequency, compared to immediate-release CD/LD,” said Robert A. Hauser, MD, professor of neurology, University of South Florida; and director, Parkinson’s Disease and Movement Disorders Center. "The duration of benefit for each dose was 70% longer with IPX203 dosed an average 3 times/day during the trial, which coudl be adjusted as needed for individual patients if IPX203 is approved."
There were no statistically significant differences in the Movement Disorder Society-Universal Parkinson Disease Rating Scale (MDS-UPDRS) Part II and Part III or Part III scores alone. Part II scores were measured during ON time and Part III scores were measured weekly.
Serious adverse events occurred in 8 participants (3.1%) taking extended-release CD/LD and 4 people (1.6%) taking immediate-release CD/LD. Treatment-emergent adverse events were reported for both study arms (108 [42.2%] for extended-release; 79 [31.6%] for immediate-release), and most commonly were nausea, dry mouth, urinary tract infection, and falling.
