Dystrophin-Expressing Chimeric Cells Show Functional Improvement in Duchene Muscular Dystrophy
Functional improvements have been observed in the first 3 individuals treated in a clinical trial of dystrophin-expressing chimeric cells (DEC)(DT-DEC01; Dystrogen Therapeutics, Miami, FL) for Duchenne muscular dystrophy (DMD). These individuals are in the low-dose cohort for this dose-finding trial. There were no adverse events associated with DEC therapy, which does not require immunosuppression and does not utilize viral vectors for cell modification.
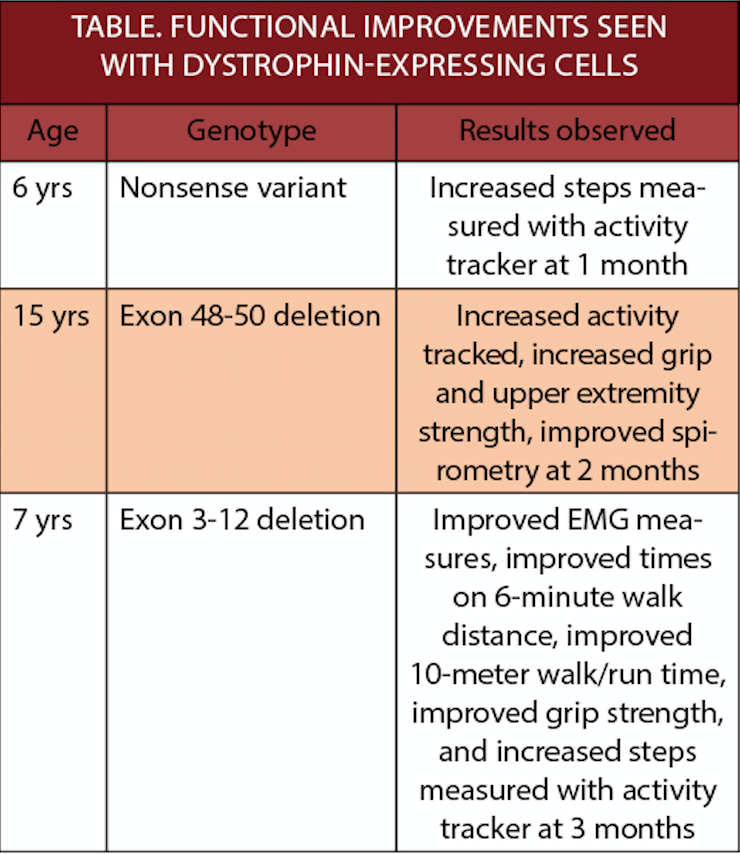
"There are currently no approved treatments for people with DMD that result in a cure or significant attenuation of the disease, which causes significant disability in (heterozygous individuals) and usually leads to early mortality. It's very encouraging that we continue to see consistent, positive data from our investigational DEC engineered cell therapy across several measures, as we know the community needs more options," said Maria Siemionow, MD PhD, chief scientific officer, Dystrogen Therapeutics. "The improvements in functional measures at 1 and 3 months in participants from the low dose cohorts who received DEC are distinctly different from what an age-matched, natural history group would predict with DMD. When coupled with the strong and sustained dystrophin expression results in preclinical studies and encouraging safety profile seen to date, today's results increase our confidence in DT-DEC01 and provide additional supportive evidence for this approach as we advance to the higher dose cohort into the next stage of clinical testing."
The Data and Safety Monitoring Board (DSMB) recommend initiating treatment in the mid-dose cohort. DEC is an engineered chimeric cell that delivers a full-length dystrophin gene and related components of a healthy muscle cell which assists with the progressive degeneration and shortened lifespan as symptoms of DMD.
