Donanemab Treatment Effects in Early Stages of Alzheimer Disease Detailed in New Post-Hoc Analysis from TRAILBLAZER-ALZ 2
According to post-hoc analyses of data from the TRAILBLAZER-ALZ 2 clinical trial (NCT04437511), people with baseline characteristics indicative of earlier stages of Alzheimer disease (AD), including lower tau PET, younger age, and plasma phosphorylated tau at threonine 217 (p-tau217) levels, showed greater benefit when treated with donanemab (Eli Lilly and Company, Indianapolis, IN) than others. The findings were presented at the 16th annual Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Post-hoc analyses were conducted for data from the over 1700 participants included in the phase 3 TRAILBLAZER-ALZ 2 clinical trial, specifically exploring 3 baseline characteristics associated with disease stage: tau PET imaging, age, and p-tau217 levels. Disease progression was assessed according to Integrated Alzheimer Disease Rating Scale (iADRS) and Clinical Dementia Rating – Sum of Boxes (CDR-SB) scores of clinical decline over 76 weeks. The following results were reported, demonstrating that participants with baseline characteristics associated with earlier disease stage showed greater treatment benefit:
- Participants treated with donanemab for 76 weeks who had low-medium tau PET imaging showed 35% and 36% slower clinical decline compared to placebo according to iADRS and CDR-SB scores, respectively.
- For participants on donanemab aged younger than 75 years who had low-medium tau PET, clinical decline was 48% and 45% slower compared to placebo according to iADRS and CDRSB scores, respectively.
- At 76 weeks, lower baseline p-tau217 levels were associated with greater slowing of clinical decline, with 36% slowing on iADRS at low p-tau217 vs 21% at high p-tau217, and 46% slowing on CDRSB at low p-tau217 vs 26% at high p-tau217.
- For participants aged younger than 75 years, lower baseline p-tau217 levels were associated with both greater treatment difference and slowing of clinical decline.
The study was sponsored by Eli Lilly and Company, who has submitted donanemab to the Food and Drug Administration (FDA) for traditional approval with regulatory action expected by the end of 2023.
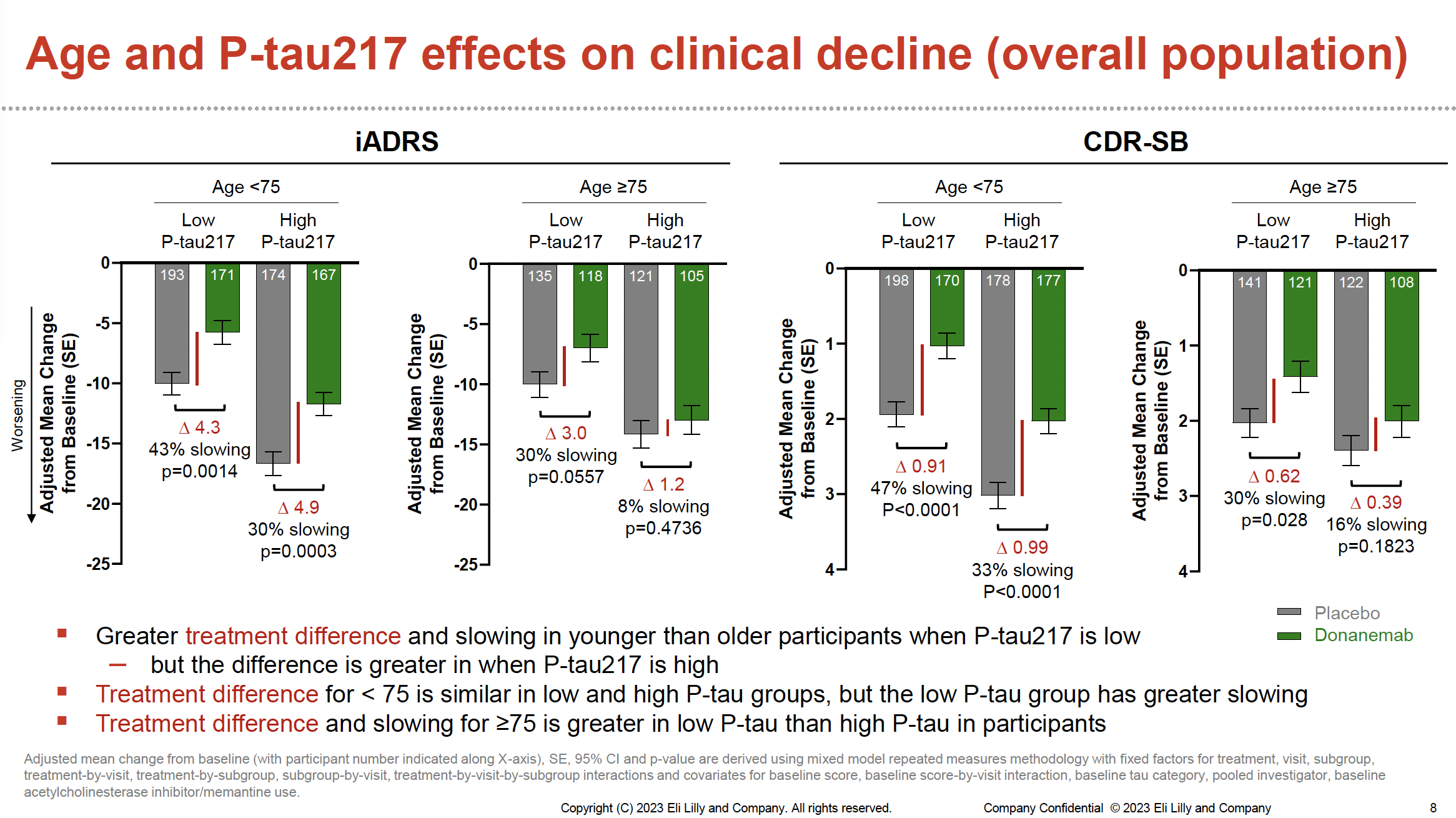
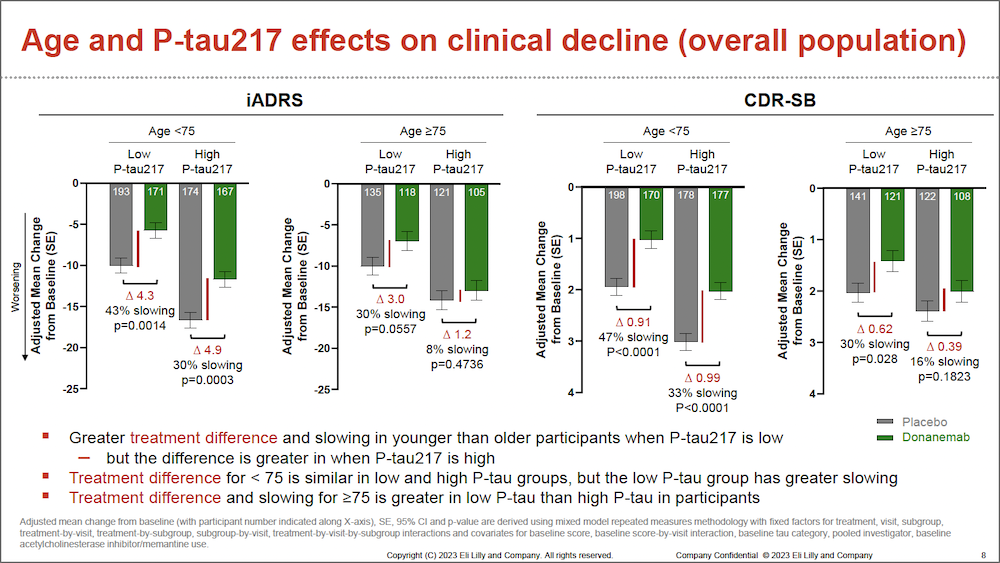
Image reproduced with permission from Eli Lilly and Company.
