Determining Optimal Treatment Strategies for Multiple Sclerosis
A review published in Lancet Neurology makes the case that well-powered randomized clinical trials to compare treatment strategies for multiple sclerosis (MS) are necessary and possible. Authored by Daniel Ontaneda, MD of the Mellen Center for Multiple Sclerosis at the Cleveland Clinic and colleagues from the US, United Kingdom, and Australia, the review is based on literature review from 2006 through 2018 and the authors’ personal literature archives.
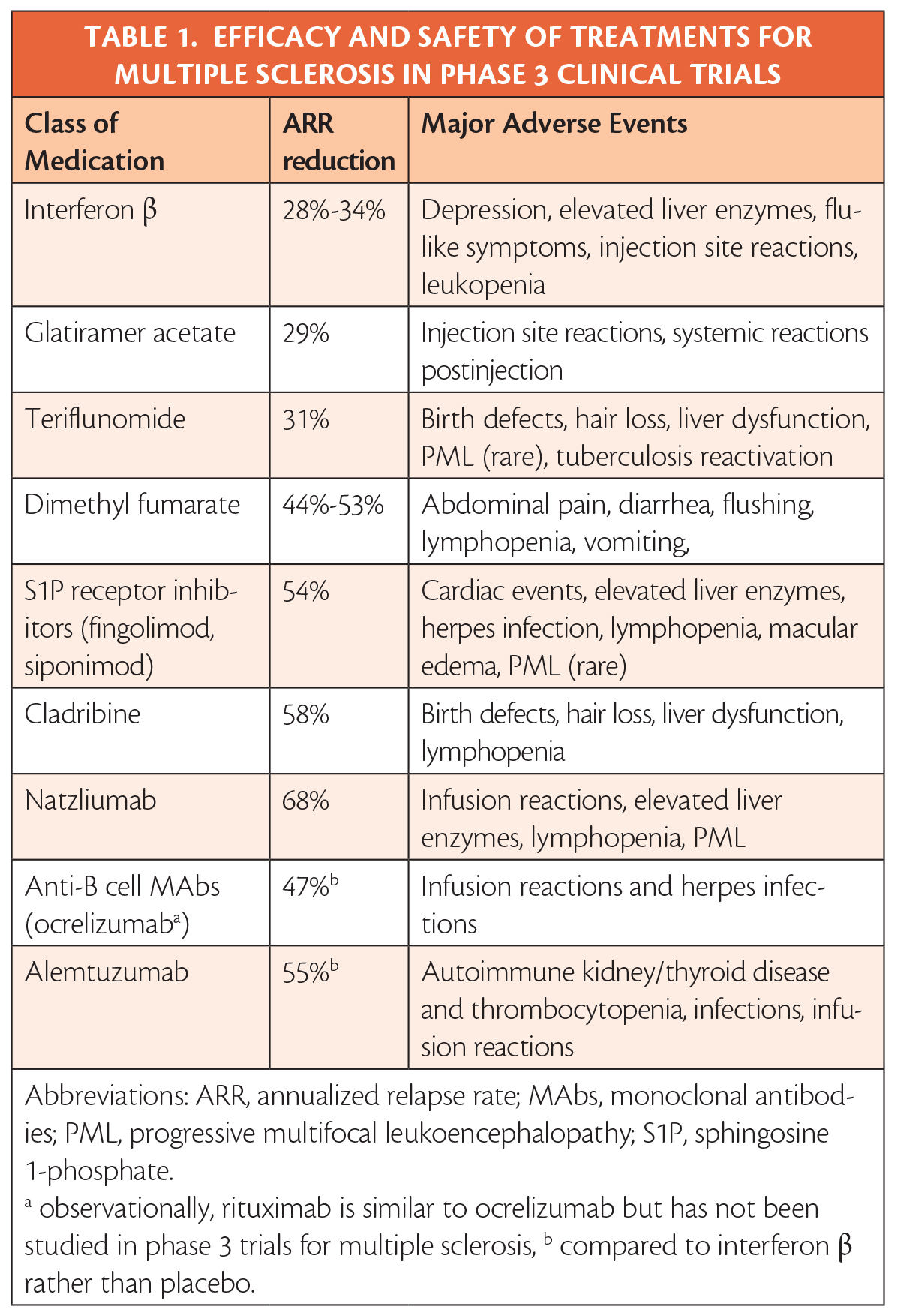
The review of the data makes clear that efficacy and safety has been established in clinical trials and allows treatments to ranked by efficacy and severity of potential adverse events (Table). It is also evident that beginning treatment at the first clinical attack has clear benefits, and that, although controversial, initiating treatment upon radiologic evidence of MS may also have benefits.
The authors note that their field is relatively privileged to have so many treatment options because of tremendous advances over the last 2 decades. They also describe the limited and primarily observational data available to guide choice of treatment both at early initiation and throughout the course of MS that results in highly variable clinical practice.
With over a dozen options in 9 classes, it is not feasible to run randomized prospective clinical trials comparing initiation of each drug vs all others. The authors make the case, however, that well-powered randomized controlled trials to compare the 2 main treatment approaches being used clinically are both possible and necessary. The first of these is escalation, which is to start treatment with moderate-effective, relatively safer treatments followed by surveillance and escalation to more highly effective treatment if/when relapse occurs. Second, is to begin early with more highly effective, albeit higher-risk treatments, switching among those if/when relapse occurs.
There are 2 trials comparing these approaches, both currently recruiting participants: DELIVER-MS (NCT03535298) and TREAT-MS (NCT03500328). The authors also encourage use of observational studies with clinical data registries of individuals who are not able to participate in controlled randomized trials.
Finally, the authors advocate that clinicians be open about what is known and where knowledge gaps still exist when discussing options with patients.
