DaxibotulinumtoxinA Improves Symptoms of Cervical Dystonia With Long-Lasting Effects
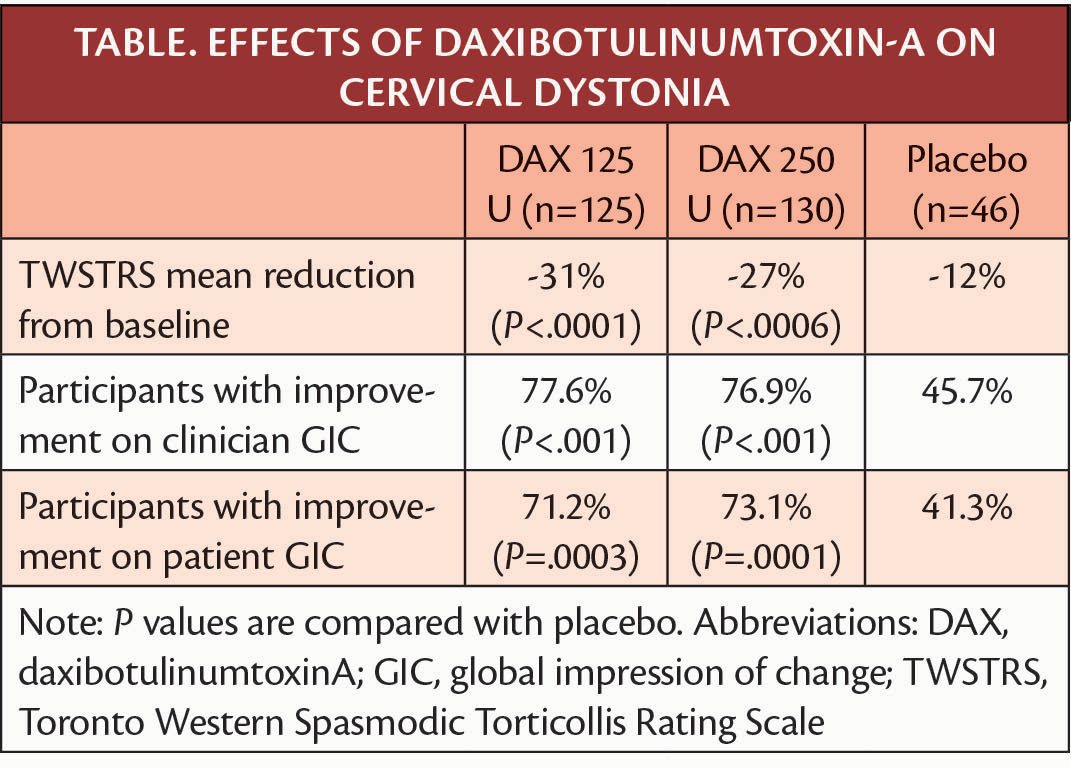
In a phase 3 clinical trial (NCT03608397) adults with moderate-to-severe cervical dystonia treated with daxibotulinumtoxinA (DAX) had statistically significant improvement in symptoms as measured by the Toronto Western Spasmodic Torticollis rating scale (TWSTRS).
"Data from the ASPEN-1 clinical trial continues to highlight the long duration of effect and encouraging safety profile of DAX for the treatment of cervical dystonia, reinforcing the drug product’s differentiated performance profile,” said Roman Rubio, senior vice president of Clinical Development at Revance. "We look forward to filing a Supplemental Biologics License Application, which may bring us one step closer to helping patients with this debilitating condition achieve long-lasting symptom relief."
Reductions seen on TWSTRS occurred across all 3 categories of the scale (ie, severity, disability, and pain), and the difference between DAX and placebo treatment was statistically significant on each subscale (P≤.175 vs placebo for all measures all doses). The study also showed the median time to loss of 80% or more of peak treatment effect was 20.3 and 24 weeks with the 125 U and 250 U doses, respectively. Participants were followed for 36 weeks, and clinical assessments were made at weeks 2, 4, 6, 12 and every 4 weeks thereafter. Change on TWSTRS reported is the least squares mean of the average of participants' scores at weeks 4 and 6.
Treatment-emergent adverse events that occurred with DAX but not placebo included swallowing difficulties and muscular weakness, although the rate of both these conditions was only 2.7% (7/255) across both DAX-treated groups.