Combination Extended Release Pramipexole/Rasagiline Effective for Parkinson Disease
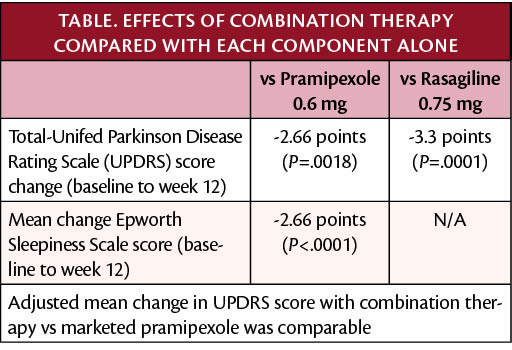
In a phase 3 study (NCT03329508), a combination therapy of pramipexole/rasagiline (P2B001; Pharma Two B, Rehovot, Israel) was superior compared with either pramipexole or rasagiline individually for treatment of Parkinson disease (PD). The combination therapy, taken orally once daily does not require titration and was more tolerable with significantly less daytime sleepiness compared with either therapy alone. Improvement in PD was measured by the change from baseline to week 12 in total Unified Parkinson Disease Rating Scale (UPDRS Part 2 and 3; primary endpoint).
Dr. Warren Olanow, Professor Emeritus of Neurology and Neuroscience at the Icahn School of Medicine at Mount Sinai in New York, commented, “The initiation of treatment of patients with PD represents an area of unmet need due to the side effects associated with current treatments. Based on the data from this well-designed, rigorous, active-controlled study, P2B001 has the potential to become a leading treatment option for PD, particularly as first-line therapy for early-stage patients of all ages. The results demonstrated both superior efficacy to components and a more favorable safety profile than treatment with standard doses of pramipexole. If approved, P2B001 would enable patients to be treated with an effective dose of a dopamine agonist, yet with less adverse events often seen with this class of drugs, including daytime sleepiness, orthostatic hypotension, and hallucinations. These issues can often negatively affect patients’ activities of daily living. P2B001 has the additional advantage of once-a-day administration without the need for titration.”
“We are thrilled with the positive outcome of this rigorous phase 3 study. There is a clear unmet medical need for an early PD treatment that can significantly improve motor symptoms and daily function, while avoiding side effects,” said Dr. Sheila Oren, MD, MBA, chief executive officer of Pharma Two B. “We would like to thank all of the study participants and investigational sites that took part in this important study”.
The combination therapy is a novel fixed-dose combination of extended release (ER) formulations of pramipexole (0.6 mg) and rasagiline (0.75 mg), with both treatments at lower doses than when given alone or together as separate concomitant treatments. Combination therapy was generally well tolerated, with more than 98% of treatment-emergent adverse events being mild or moderate in severity.
