Children Treated With Risdiplam Continue Meeting Motor Milestones After 1 Year
Results from Part 2 of a pivotal clinical study show that infants with symptomatic Type 1 spinal muscular atrophy (SMA) treated with risdiplam (Evrysdi; PTC Therapeutics, South Plainfield, NJ) showed significant improvements in survival, motor milestones, and motor function compared with the natural history of the disease.
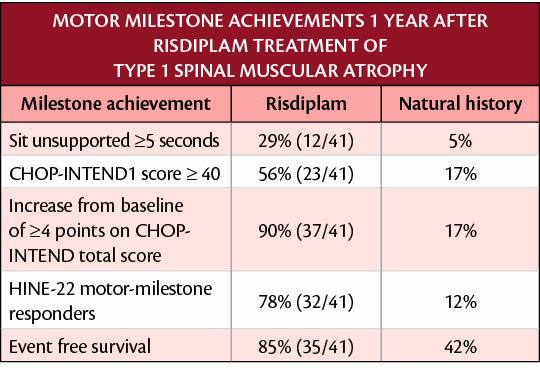
The table below depicts the primary and secondary endpoints achieved after 12 months of treatment with risdiplam in the FIREFISH trial (NCT02913482).
“It is exciting to see such profound results in infants treated with Evrysdi,” said Stuart W. Peltz, PhD, chief executive officer, PTC Therapeutics. “We are proud that such a significant treatment for SMA patients was developed from PTC’s splicing platform. We believe that the splicing platform is a disruptive technology that will continue to drive the development of new therapies in diseases with no treatment options available.”
The safety profile of Evrysdi was established across pivotal trials. The most common adverse reactions in later-onset SMA (incidence of at least 10% of patients treated with Evrysdi and more frequently than control) were fever, diarrhea, and rash. The most common adverse reactions in infantile-onset SMA were similar to those observed in later-onset SMA patients. Also, the most common adverse reactions (incidence of at least 10%) were upper respiratory tract infection, pneumonia, constipation, and vomiting.
FIREFISH is an open-label, 2-part pivotal clinical trial in infants with Type 1 SMA. Part 1 was a dose-escalation study in 21 infants with the primary objective of assessing the safety profile of risdiplam in infants and determining the dose for Part 2.
Part 2 is a pivotal, single-arm study of risdiplam in 41 infants with Type 1 SMA treated for 2 years followed by an open-label extension. The primary objective of Part 2 was to assess efficacy as measured by the proportion of infants sitting without support after 12 months of treatment, as assessed in the Gross Motor Scale of the Bayley Scales of Infant and Toddler Development—Third Edition (BSID-III) (defined as sitting without support for 5 seconds).
