Cenobamate Treatment Reduces Partial-Onset Seizure Frequency in a Durable, Persistent, and Safe Manner
Cenobamate (Xcopri; SK Life Sciences, Paramus, NJ) was approved by the Food and Drug Administration (FDA) in November 2019 for treatment of partial-onset seizures in adults. In a phase 2 double-blind placebo-controlled trial (NCT01866111), treatment with 200 mg or 400 mg of cenobamate reduced seizure frequency by 55% compared with 24% for placebo (P<.01). Seizure freedom was achieved by 11.2% and 21.1% of participants treated with 200 mg or 400 mg cenobamate, respectively, vs 1.0% for people treated with placebo.
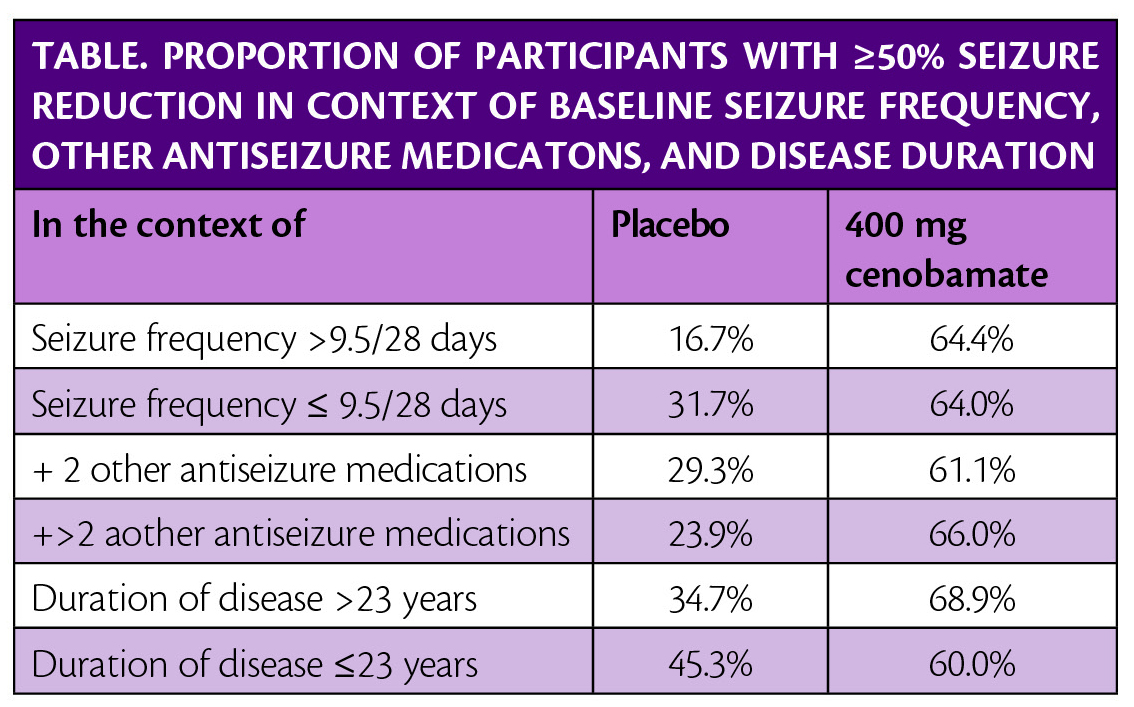
Post hoc analysis of the study, presented at the American Epilepsy Society Annual Meeting in Baltimore, MD December 6-10, 2019, shows that seizure frequency reductions were not dependent on the number of concomitant antiseizure medications (ASMs). Seizure frequency reduction was also not dependent on baseline seizure frequency or duration of epilepsy (Table).
Analysis of safety data from this trial, another phase 2 double-blind placebo-control trial (NCT01397968) and a long-term open-label extension study (NCT02535091) were also reported. Of the 1,944 participants in the trials, 1,525 had cenobamate titrated weekly and 419 had cenobamate titrated every 2 weeks. A smaller percentage of those who had biweekly titration had serious treatment-emergent adverse events (TEAEs) compared with those who had weekly titration. Seizure, dizziness, and vertigo were the only serious TEAEs occurring in at least 1% of participants who had biweekly titration. A single participant taking 200 mg/day of cenobamate who had weekly titration experienced a serious TEAE of drug reaction with eosinophilia and systemic symptoms (DRESS) on treatment day 24; no other cases of DRESS occurred.
In the open-label extension period for NCT01397968, 71% of participants continued on cenobamate at 1 year, and of those who had continued for 1 year, 81% continued for a median duration of 5 years.
Marc Kamin, MD, of SK Life Sciences said, “Cenobamate has encouraging response rates and up to 20% of people who took it at higher doses in clinical trials achieved seizure freedom. Patients who continued treatment at those doses saw similar outcomes. Just as important is our data showing that cenobamate is safe when started and titrated as described on the label.”
Treatment with cenobamate should begin at 12.5 mg/day and titrated by doubling the dose every 2 weeks until a therapeutic effect or total dose of 100 mg/day is reached. If needed, additional escalations to 150 mg/day, 200 mg/day, or a maximum dose of 400 mg/day can be used.
