Cenobamate Provides High Rates of Seizure Freedom and Reductions or Discontinuations of Concomitant Antiseizure Medications
Cenobamate was approved for treatment of partial onset seizures in May 2020 (Xcopri; SK LIfe Science, Paramus, NJ), and results of open-label extension studies have now been reported. Seizure reduction rates in the 214 people who had at least 1 dose of cenobamate after a 12-week titration period are shown in the Table.
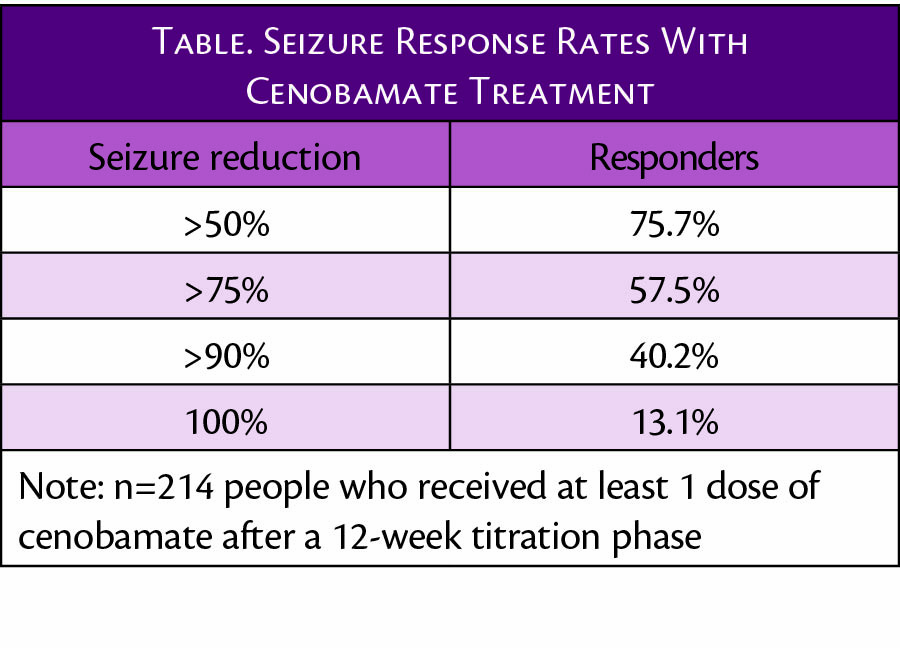
Of note, of 240 individuals who began the open-label study, 74.8% continued taking cenobamate, and 36.3% achieved complete seizure freedom for at least 1 period of 12 consecutive months or more.
Dr. Michael Sperling, Baldwin Keyes Professor of Neurology at the Sidney Kimmel School of Medicine, Thomas Jefferson University, Philadelphia, PA said, "The high rates of continuation and response in this trial—with 3 out of 4 people continuing Xcopri for over 2.5 years and 1 in 3 of those achieving seizure freedom for at least a year—suggest a sustained and robust long-term response to treatment with Xcopri. As real-world safety data and continued use support the safety and efficacy, I expect Xcopri will provide many people with epilepsy an effective and safe treatment option that may deliver freedom from seizures for extended periods."
Dose reductions or discontinuations of concomitant ASMs were allowed and occurred primarily between weeks 8 and 29 of starting cenobamate. Of 395 concomitant medications, 24.8% (98/395) were discontinued. People who continued taking cenobamate had larger dose reductions for 80% of concomitant ASMs compared with those who stopped cenobamate. Notably, this includes clobazam, which has increased blood levels (of active metabolite) when administered with cenobamate, and carbamazepine and lamotrigine, even though pharmacokinetic studies had suggested blood levels of these drugs might be lowered with concomitant cenobamate. The most common reasons given for reducing or discontinuing a concomitant ASM were fatigue, somnolence, dizziness, and unsteady gait.
In early clinical trials, 3 cases of drug-induced hypersensitivity syndrome (DRESS) occurred, but none have been seen in later trials or real-world use in over 5,000 people using a low and slow titration initiated with 12.5 mg/day and increased over 12 weeks to 200 mg/day with maximum dosage up to 400 mg/day if needed.
These data were presented at the virtual 2021 American Academy of Neurology (AAN) Virtual Annual Meeting April 17-22, 2021.
