Can -Gepants Do It All For Migraine?
The -gepants are a new class of small molecule antagonists of calcitonin gene-related peptide receptor (CGRP) medications. There is growing evidence that, like the monoclonal antibodies to CGRP or its receptor, the -gepants can prevent migraine when taken regularly. There are 2 -gepants already approved for acute treatment of migraine—ubrogepant (Ubrelvy; Abbvie, Madison, NJ) and rimegepant (Nurtec; Biohaven, New Haven, CT) and 2 others in development—zavegepant (Biohaven) a nasal spray formulation for acute treatment and atogepant (Abbvie).
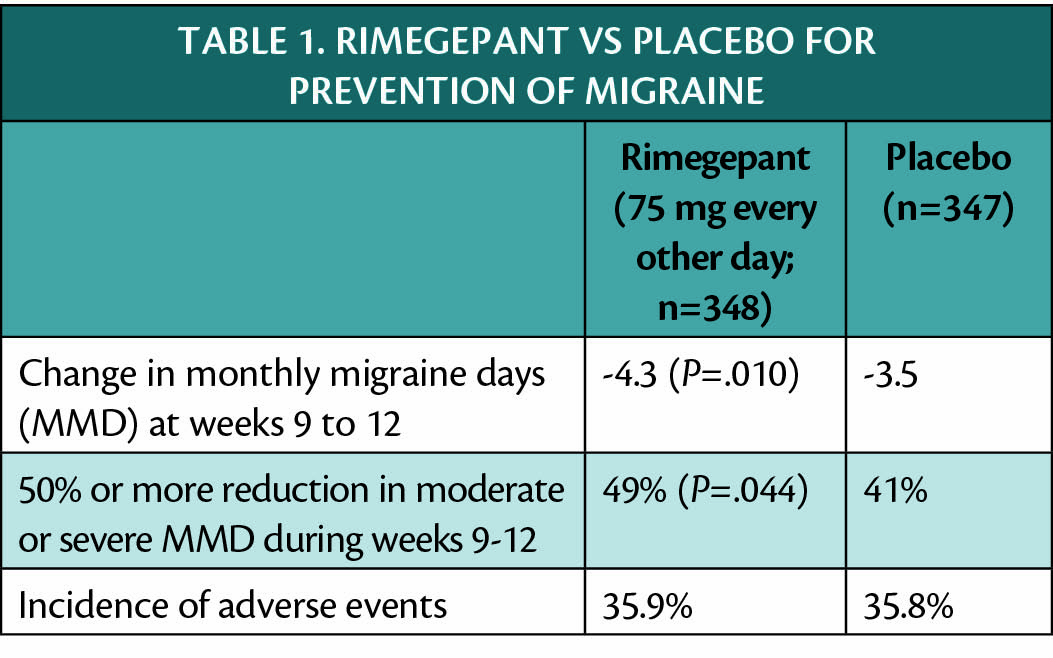
Rimegepant has also been shown effective for migraine prevention when taken orally every other day, and an application for approval of this indication has been submitted to the Food and Drug Administration (FDA). Data from a multicenter double-blind placebo-controlled trial (NCT03732638) of rimegepant for preventive treatment of migraine are shown in Table 1.
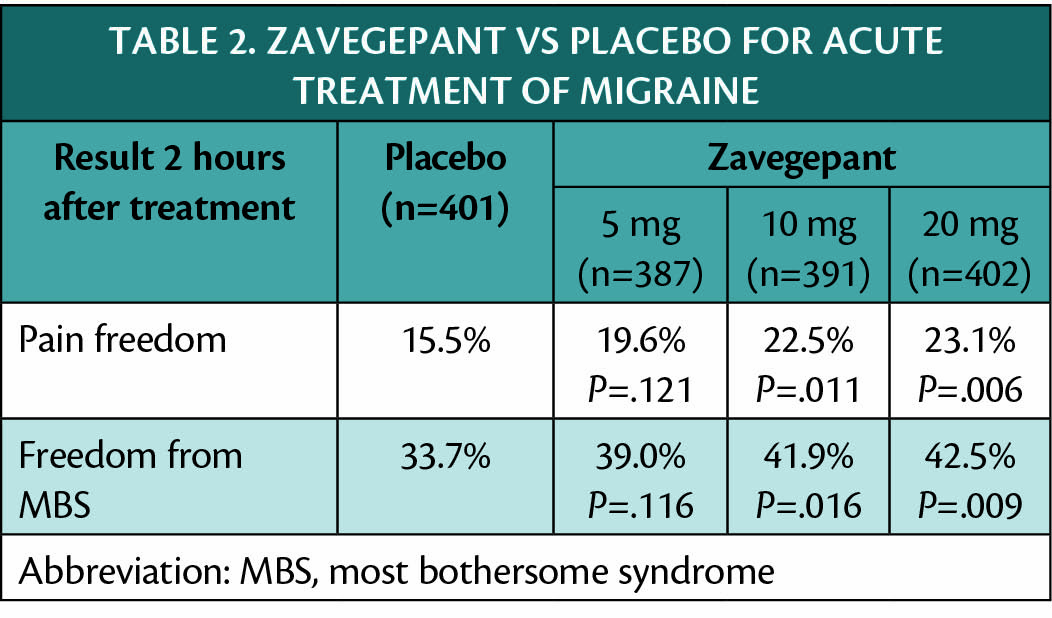
In addition, another acute treatment zavegepant (Biohaven) is the only intranasal CGRP receptor antagonist in development and has also shown efficacy in a large clinical trial (NCT03872453) as shown in Table 2. Adverse events of zavegepant included dysgeusia (13.5%-16.1% with zavegepant vs 3.5% with placebo) and nasal discomfort (1.3%-5.2% with zavegepant vs 0.2% with placebo). There was no signal of hepatoxicity.
David Kudrow, MD, director of both the California Medical Clinic for Headache and the Headache Clinic Harbor-UCLA Medical Center and assistant clinical professor in Neurology at the UCLA David Geffen School of Medicine, said "These results show that rimegepant, which is already approved in the US for the acute treatment of migraine, is also effective as a preventive treatment for migraine. What makes this rimegepant data particularly interesting is that it challenges our traditional understanding of the difference between acute and preventive treatment. The intranasal zavegepant data shows superior efficacy to placebo and introduces a route of delivery unique to the CGRP antagonist class."
Participants in the trial of zavegepant (NCT03732638) were mean age 40 years, 85.5% female persons, and approximately 14% were taking preventive migraine medication.
Participants in the migraine prevention trial of rimegepant (NCT03872453) were adults with a history of 4 to 18 migraine attacks of moderate-to-severe pain intensity per month; the study population had mean age 41 and was 83% female persons and 82% white persons. The rate of adverse events in people treated with rimegepant was similar to that of people treated with placebo and adverse events were generally mild and transient.
These data were presented at the virtual 2021 American Academy of Neurology (AAN) Virtual Annual Meeting April 17-22, 2021.
