Autologous Mesenchymal Stem Cells Expressing Neurotrophic Factors Improved Function and Biomarkers in Progressive Multiple Sclerosis
In a trial (NCT03799718) of autologous neurotrophic mesenchymal stem cells (MSC-NTF) (NurOwn; BrainStorm Cell Therapeutics, New York, NY), 18 individuals with progressive MS were treated, and 16 completed the trial. Results of treatment were compared with 48 case-matched controls from the Comprehensive Longitudinal Investigation of Multiple Sclerosis (CLIMB) registry (CLIMB) and the participants in the NN-102 SPRINT-MS Study (NCT01982942) treated with placebo.
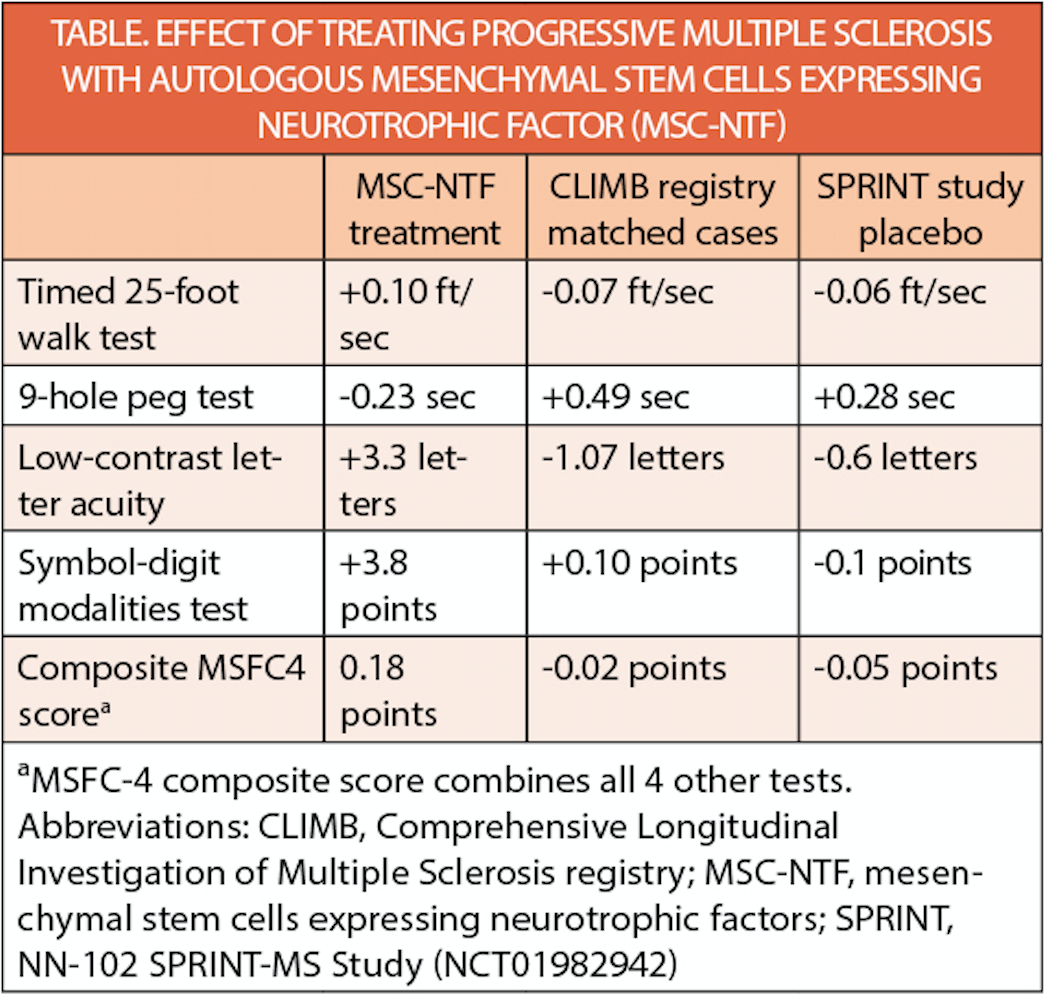
On the T25W test, 14% of participants treated with MSC-NTF had had at least a 25% improvement in the timed 25-foot walk (T25FW) test compared with 5% and 9% in CLIMB and STRIVE respectively. For the 9-Hole Peg Test (9-HPT), 13% of those who had MSC-NTF treatment had 25% improvement vs 0% and 3% of those in CLIMB and STRIVE, respectively. With MSC-NTF treatment, 27% of participants had at least an 8-letter improvement in low contrast letter acuity (LCLA) and 67% had a 3-point or more improvement on the Symbol Digit Modalities Test (SDMT), compared with 6% and 18% in CLIMB and 13% and 35% in SPRINT. On the 12-item MS Walking Scale (MSWS-12), 38% of participants treated with MSC-NTF had at least a 10-point improvement. The MSWS-12 was not evaluated in CLIMB or SPRINT.
Cerebrospinal fluid (CSF) biomarkers measured at 3 consecutive time points showed increases of neuroprotective molecules (vascular endothelial growth factor [VEGF], hepatocyte growth factor [HGF], neural cell adhesion molecule 1 [NCAM-1], follistatin, and fetuin-A). Neuroinflammatory biomarkers (monocyte chemoattractant protein-1 [MCP-1], stromal cell-derived factor 1 [SDF-1], sCD27 and osteopontin) decreased.
“We were pleased that this study demonstrated safety, preliminary evidence of efficacy and relevant biomarker outcomes in patients with progressive multiple sclerosis, in an area of high unmet need,” said Jeffrey Cohen, MD, director of Experimental Therapeutics at the Cleveland Clinic Mellen Center for MS and principal investigator for the trial. “These results should be confirmed in a randomized placebo-controlled trial.”
In the study, the average age of participants was 47 years and had an average baseline EDSS score was 5.4. Data results were compared with matched (n=48) participants, 56% were women, in the CLIMB registry and individuals in the SPRINT study who received placebo. Out of the 18 participants treated, 80% received all treatments and completed the study. The study discontinuations were due to procedure-related adverse events with no deaths or treatment-related adverse events due to worsening of MS observed.
