Atogepant Significantly Reduced Monthly Migraine Days for Chronic Migraine
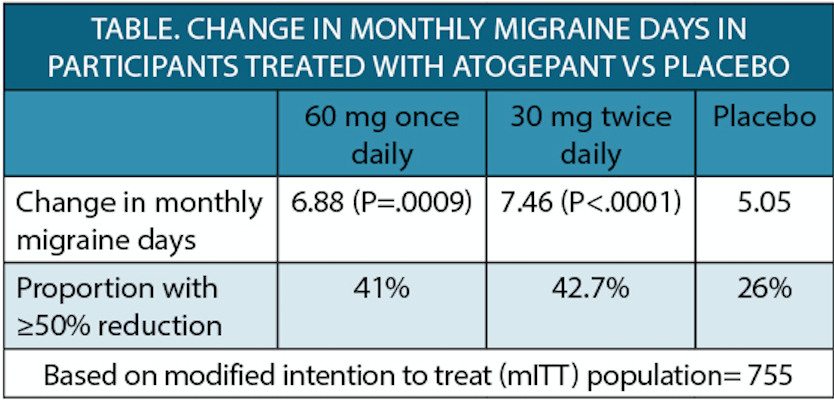
Atogepant (Quliptatm; AbbVie, North Chicago, IL), 60 mg once daily or 30 mg twice daily , significantly reduced monthly migraine days (MMD) compared with placebo in phase 3 trial (NCT03855137) for adults with chronic migraine. Participants (n=778) who had at least a 1-year of chronic migraine were randomly assigned to receive atogepant or placebo treatment over a 12-week period.
“AbbVie has nearly 12 years of experience in treating chronic migraine, a debilitating disease. We know that no 2 migraine patients are alike, so it is important for health care providers to have a variety of treatment options,” said Michael Severino, MD, vice chairman and president, AbbVie. “These data and pending regulatory submissions solidify our commitment to our leading migraine portfolio to help the more than one billion people worldwide living with the migraine. We look forward to taking the next steps to potentially expand the use of atogepant in the US to include the preventive treatment of chronic migraine in adults, and to working with regulatory agencies globally on additional submissions.”
The most common adverse events reported with a frequency ≥ 5% were constipation and nausea in mild or moderate severity. None of these treatment-emergent adverse events were assessed as treatment-related by the investigator.
