Atogepant Shows Efficacy for Prevention of Migraine and Improved Function
Daily treatment with atogepant, which is still investigational, has shown efficacy for migraine prevention in a phase 3 clinical trial (NCT03777059) as shown in the Table.
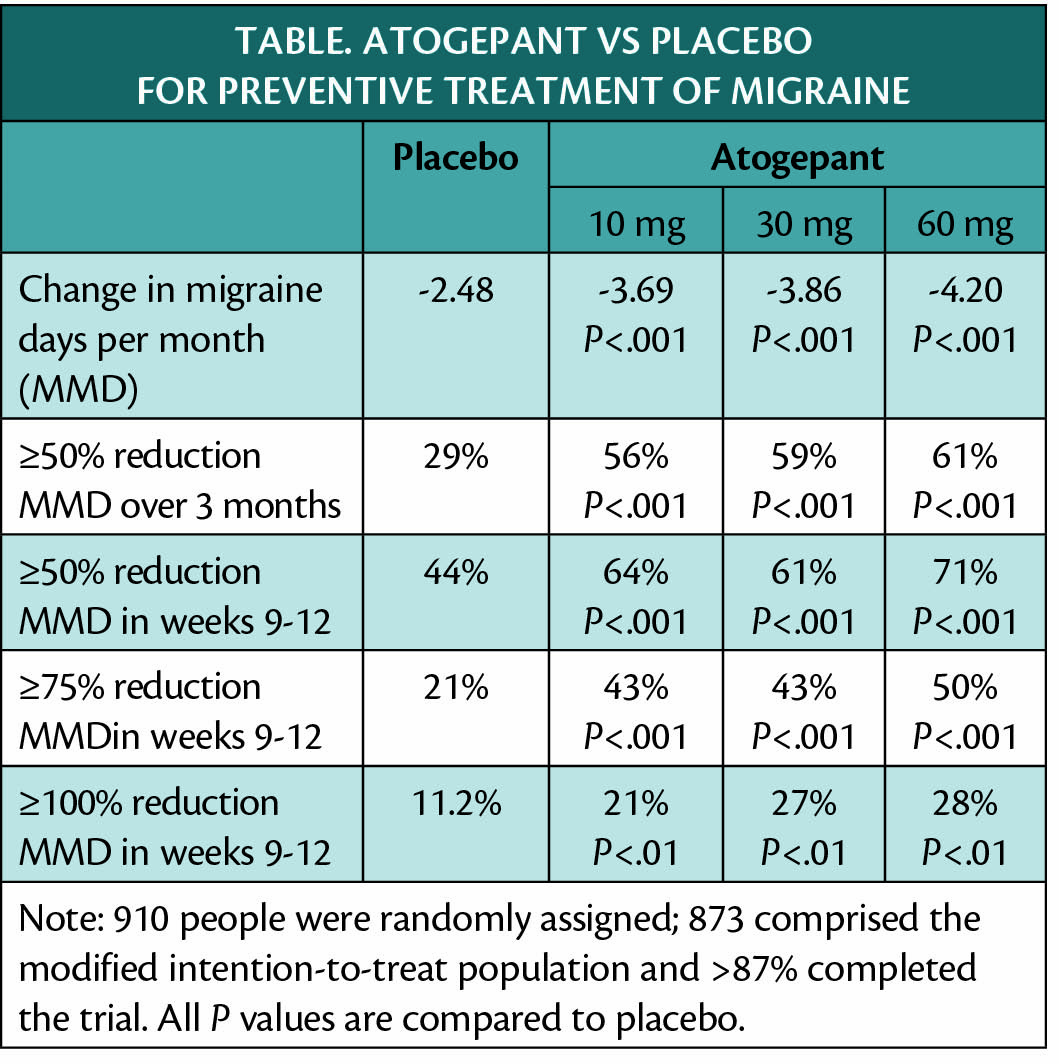
In prespecified analysis, it was found that the effect of atogepant on reducing monthly migraine days (MMD) began as early as the first week of treatment. Use of acute medications, number of days with a headache were reduced with atogepant at all doses compared with placebo (P<.001).
Participants in the phase 3 double-blind trial (NCT03777059) of atogepant were adults who experienced 4 to 14 migraine days per month Participants were mean age 42 with an average body mass index (BMI) 31kg/m2. Women comprised 89% of the participant group, and 83% of participants were white.
Individuals treated with atogepant 30 or 60 mg also had statistically significant improvements on multiple patient-reported outcomes including the Performance of Daily Activities (PDA) and Physical Impairment (PI) domains of the Activity Impairment in Migraine-Diary (AIM-D), and the Headache Impact Test (HIT-6).
Adverse events that were higher in people treated with atogepant vs placebo were constipation (7%-8% across doses vs 0.5% placebo) and nausea (4%-6% across doses vs 2% placebo), and discontinuation rates in people treated with atogepant were 2% to 4%. No hepatic safety issues were observed.
Additionally, in an open-label trial (NCT03700320), adult participants were randomly assigned 5:2 to receive atogepant 60 mg/day or another standard-of-care migraine preventive medicine. Of those treated with atogepant, 94% continued treatment for over 1 year. Elevated transaminases were seen in 2.4% (13/531) of people who took atogepant and 3.2% (6/190) of those who had standard-of-care treatment.
These data were presented at the virtual 2021 American Academy of Neurology (AAN) Virtual Annual Meeting April 17-22, 2021.
