Atogepant for Prevention of Episodic Migraine-- Primary Outcome Measure Met in Phase 2b/3 Clinical Trial
Results of a phase 2b/3 clinical trial (NCT02848326) show that atogepant (Allergan, Madison, NJ) treatment significantly reduced the mean number of migraine days over a 12-week period for people with migraine, meeting the trial’s primary efficacy endpoint (Table).
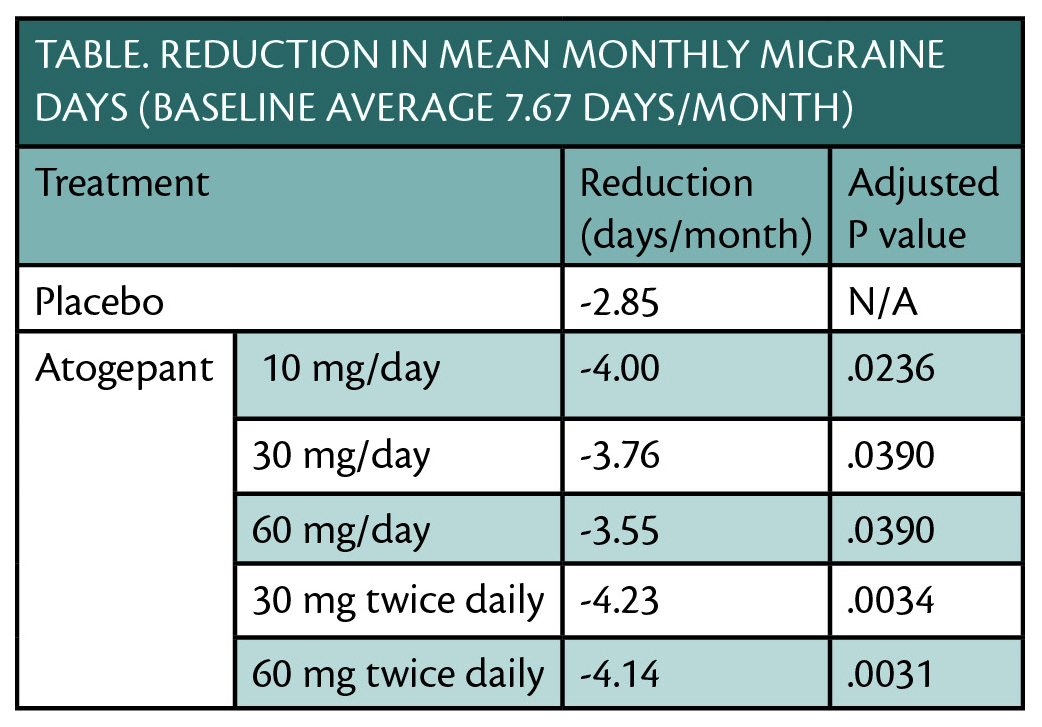
Atogepant was well tolerated and no treatment-related serious adverse events occurred. Reductions in monthly migraine days were seen as early as weeks 1-4 and were sustained over 12 weeks of treatment. Atogepant is a small molecule calcitonin gene-related peptide (CGRP) receptor antagonist that is orally available.
In exploratory analysis of the data from this trial, the proportion of patients achieving different response rates (25%, 50%, 75%, and 100%) were compared to the proportion of participants with that response rate from placebo. Treatment with any of the 5 doses of atogepant vs placebo resulted in a significantly larger proportion of participants achieving the response rate measured (25% response: 71%-84% vs 63%, 50% response: 52%-62% vs 40%, 75% response 29%-37% vs 18%, and 100% response: 7%-11% vs 3%).
These results were presented at the American Headache Society’s 61st Annual Meeting July 11-14 in Philadelphia PA.
In a late-breaking presentation at the meeting, it was shown that in animal models extracranial injections of onabotulinumtoxinA (Botox; Allergan, Madison, NJ) in combination with intravenous injection of atogepant attenuates activation and sensitization of c-fiber, high-threshold (TH) and A-delta, wide dynamic range (WDR) pathways. These findings suggest that a combination therapy that blocks both these pathways may be more effective than a monotherapy that blocks only one of them.
