Apitegromab Treatment Provides Motor Function Improvement for Nonambulatory Individuals with Spinal Muscular Atrophy Type 2 and 3
In the open-label phase 2 TOPAZ trial (NCT03921528), nonambulatory individuals with spinal muscular atrophy (SMA) treated with apitegromab (ScholarRock, Cambridge, MA) for 24 months had sustained and continued motor function improvements.
Participants were age 2 to 21 years and had SMA type 2 or 3. Those with valid Hammersmith Functional Motor Scale Expanded (HFMSE) assessments maintained sizeable improvement from their pretreatment baseline that was seen after 12 months of treatment. Continued improvements in upper limb function occurred as measured with the Revised Upper Limb Module (RULM) The mean change from baseline results for non-ambulatory patients showed:
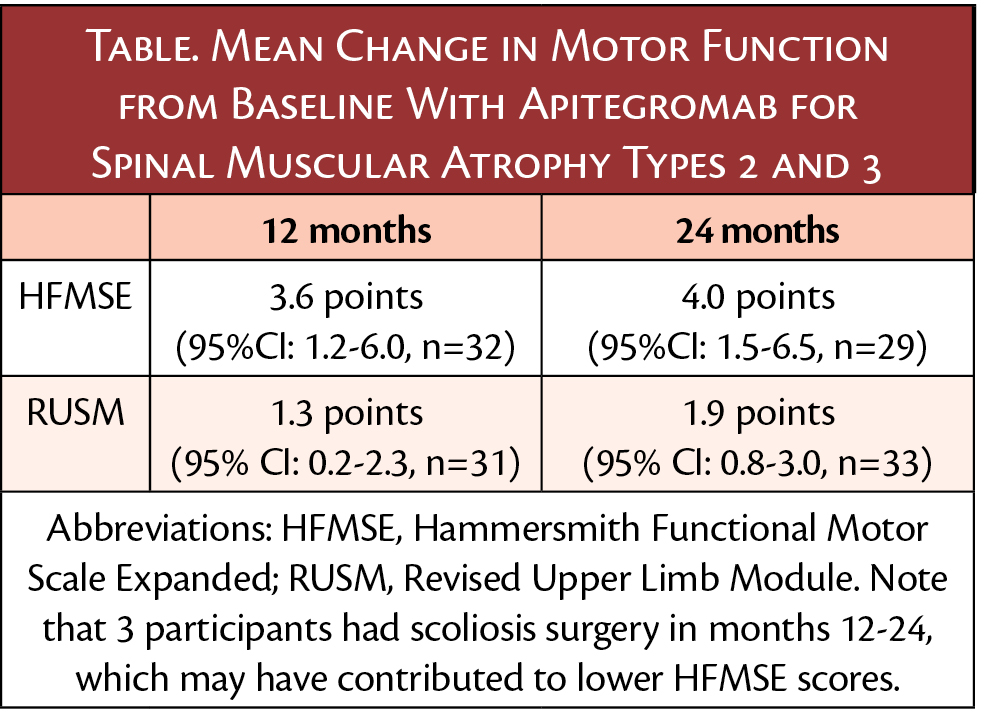
"The 24-month results provide long-term data and evidence, underscoring the findings of the 12-month primary treatment period of the TOPAZ trial in which patients receiving apitegromab experienced sizable motor function gains,” said George Nomikos, MD, PhD, senior vice president of clinical sciences, ScholarRock. “This durability and continued increase in motor function support the transformative potential of apitegromab for patients suffering with SMA.”
Consistent with the 12-month safety data, no serious safety risks were identified as part of the analysis of the cumulative 24-month data. The incidence and severity of adverse events were consistent with the underlying patient population and background therapy.
Participants were treated with apitegromab intravenously every 4 weeks as monotherapy or with nusinersen, which was administered intrathecally.
