Aducanumab Treatment in Clinical Trials Lowered Participants' Levels of Potential Blood Biomarker for Alzheimer Disease
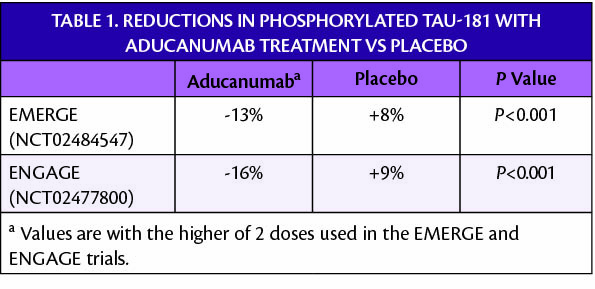
Data from blood plasma samples taken from participants in clinical trials of aducanumab (Aduhelm; Biogen, Cambridge, MA and Eisai, Woodcliffe Lake, NJ) show correlations between changes in plasma levels of phosphorylated tau 181 (Ptau-181) and other outcome measures. Decreased Ptau-181 correlated with dose and duration of aducanumab treatment. Ptau-181 is a potential blood biomarker for the presence of Alzheimer disease (AD) pathology.
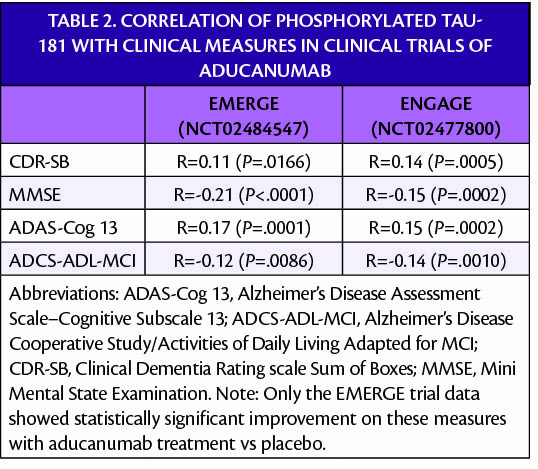
Abbreviations: ADAS-Cog 13, Alzheimer’s Disease Assessment Scale–Cognitive Subscale 13; ADCS-ADL-MCI, Alzheimer’s Disease Cooperative Study/Activities of Daily Living Adapted for MCI; CDR-SB, Clinical Dementia Rating scale Sum of Boxes; MMSE, Mini Mental State Examination.
Note: Only the EMERGE (NCT02484547) trial data showed statistically significant improvement on these measures with aducanumab treatment vs placebo.
Changes in plasma Ptau-181 also significantly correlated with changes in amyloid beta (Aβ positron emission tomography (PET) standardized uptake value ratio (SUVR) in EMERGE (R=.38, P<.0001) and ENGAGE (R=.42, P<.0001).
“These data not only show an important link between the ability of Aduhelm to clear Aβ plaque and reduce plasma p-tau levels, but also significantly correlate those reductions with slowing cognitive decline,” said Oskar Hansson, MD, PhD, professor of Neurology at Lund University and Skåne University Hospital, Sweden. “Having research from nearly 2,000 patients provides invaluable insights into the dynamics of the interconnected pathologies within this complex disease.”
This prespecified analysis was conducted by an independent laboratory. More than 7,000 blood samples from over 1,800 participants were evaluated for the Ptau-181 plasma data. These findings were presented today at the Clinical Trials on Alzheimer’s Disease conference (CTAD), held November 9-12 virtually and in Boston, Massachusetts.
