Abbott’s Portable Whole Blood Test for TBI Cleared by FDA
Abbott (Chicago, IL) announced that the FDA has granted 510(k) clearance to the i-STAT TBI cartridge. The portable i-STAT TBI cartridge system enables whole blood testing for suspected traumatic brain injury (TBI), providing lab-quality results to physicians within 15 minutes. The device, which is cleared for adults aged 18 years and older, is intended to be used by physicians in conjunction with other clinical information in the evaluation of patients with suspected mild traumatic brain injury (mTBI) and for ruling out the need for CT scans of the head.
To use the system, a small venous blood sample is applied to the i-STAT TBI cartridge, which is then inserted into a portable i-STAT Alinity instrument. The device then provides whole blood analysis for 2 biomarkers of TBI: ubiquitin C-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP). According to a statement from Abbott, the device can be used within 24 hours of injury, potentially advancing the speed of diagnosis and treatment for people who have sustained head trauma. The device is portable and can be used in settings outside the emergency department of a hospital.
“Healthcare providers can now use a rapid blood test to gather crucial information quickly,” said Beth McQuiston, MD, RD, Medical Director of Abbott Laboratories. “It’s a tremendous advance for the efficiency of emergency room operations and overall patient care and safety. It’s a significant advancement in our standard of care for traumatic brain injuries.”
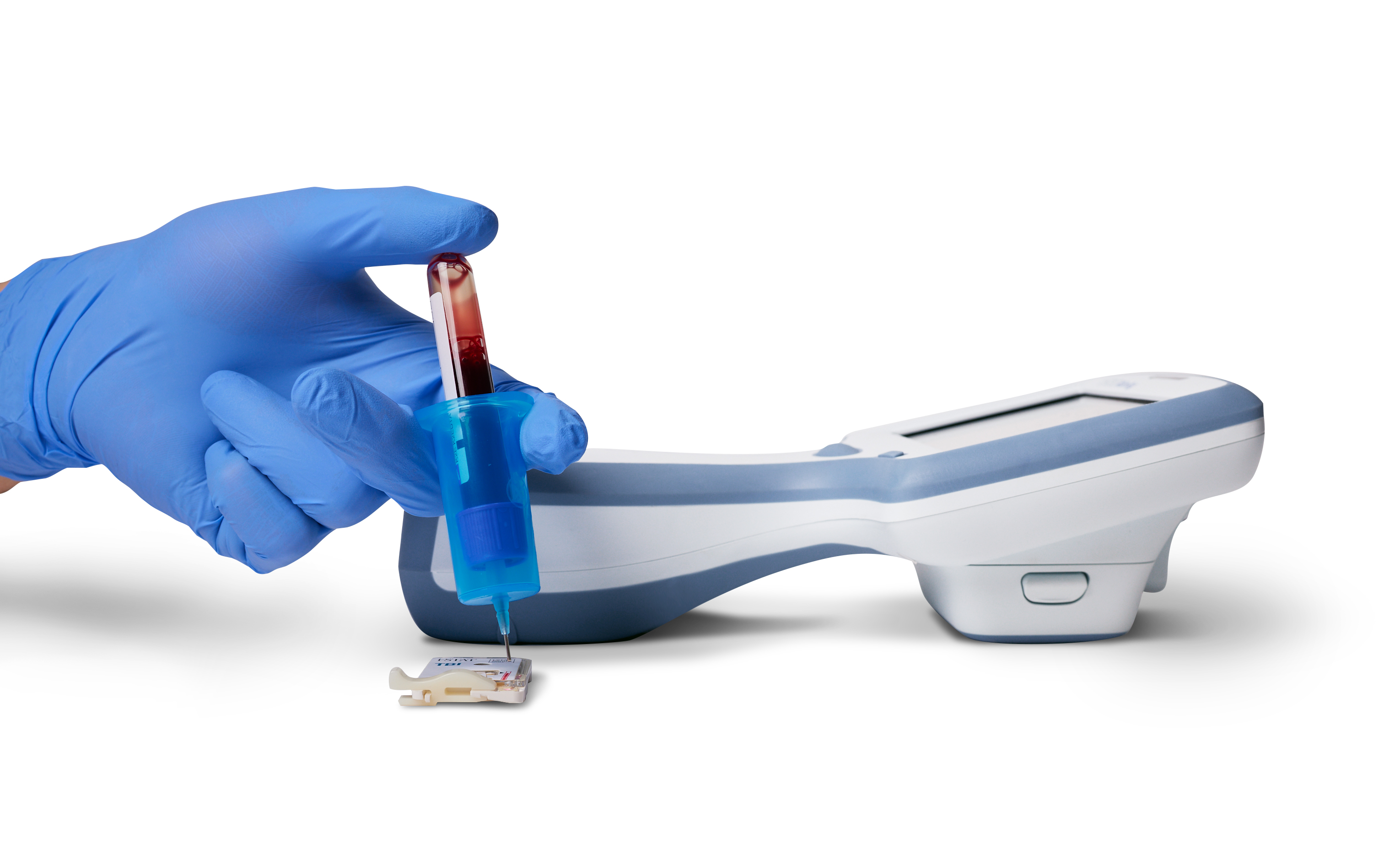
A whole blood sample is applied to an i-STAT TBI cartridge, next to the i-STAT Alinity device.
