Cenobamate Treatment Results in Sustained and Meaningful and Profound Seizure Reduction Across Multiple Partial-Onset (Focal) Seizure Subtypes
In new post-hoc retrospective analyses of long-term studies of antiseizure medication (ASM) cenobamate (Xcopri; SK Life Science, Paramus, NJ) for treatment of partial-onset (focal) seizures, 74% (n=177) of participants have continued use for a median 30.2 months. High rates of clinically meaningful seizure frequency reduction were seen across multiple subtypes of partial-onset (focal) seizures.
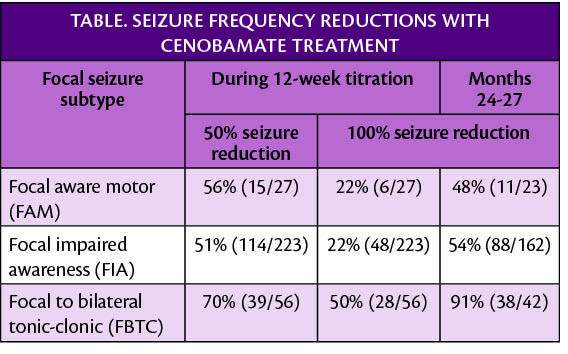
In a subset of 85 participants who had previous epilepsy surgery (54 had resection, 45 had VNS or RNS; total >85 because 14 had both) and had a mean 26 seizures/month, 31% (26/85) maintained seizure freedom for at least 12 consecutive months while being treated with cenobamate (median exposure ~2.5 years).
A separate analysis showed reductions in seizure frequency were sustained over 2.5 years of follow-up whether they had less than 3 seizures/month or 3 or more seizures/month and for those who had seizures resistant to surgical treatment. Additionally, an indirect treatment comparison study was presented that suggests cenobamate may provide higher odds of 50% or more seizure reduction compared with brivateracetam, eslicarbazepine, lacosamide, or perampanel as a group (P<.037). Previous research has also shown that those treated with cenobamate are able to lower doses of or discontinue concomitant ASMs with improved tolerability, reduction in concomitant drug load, continued improvement in seizure reduction and/or seizure freedom, and little or no negative effect on seizure frequency.
Together, these data suggest that cenobamate trials are likely to be of benefit for persons with treatment-resistant epilepsy and that a trial of cenobamate may be warranted prior to surgical treatments for epilepsy.
“Xcopri provides early, and often profound, reduction in seizure frequency—often early during titration, and benefits of Xcopri are sustained over time. This is potentially life-changing for our patients and may be a “game-changer” for the field," said William E. Rosenfeld, MD, epileptologist/neurologist, principal investigator at the Comprehensive Epilepsy Care Center for Children and Adults in St. Louis, Missouri. “Based on these findings, Xcopri may be an option for patients both early in their treatment regimen as well as later on, if prior treatments – including surgery – have been unsuccessful.”
The most common treatment-emergent adverse events were fatigue, dizziness, and somnolence, consistent with other studies of cenobamate. No occurrences of drug rash with eosinophilia and systemic symptoms (DRESS) were seen in the C021 post hoc analyses described or as far as SK Life Sciences is aware in real world experience with almost 16,500 individuals now having been treated.
These data were presented at the American Epilepsy Society (AES) Annual Meeting, held December 3-7, 2021 in Chicago, Illinois and virtually through AES 2021 Digital Select.
