The Approval of PFO Closure in the United States
Article reprinted, with updates from recently published clinical trials, with permission from the May/June 2017 edition of Cardiac Interventions Today.
In October 2016, the US Food and Drug Administration (FDA) approved the use of the Amplatzer PFO occluder (St. Jude Medical, Inc.) for percutaneous closure of patent foramen ovale (PFO). The Amplatzer PFO occluder became the first device commercially available in the United States for use in patients with presumed PFO-mediated stroke. This approval for recurrent stroke prevention capped a 16-year journey in the United States in which several devices have been used, some under a humanitarian device exemption (HDE) and some as part of randomized clinical trials. The approval has generated enthusiasm among all of the various stakeholders, as it fulfills an important unmet clinical need. Patients diagnosed as having a stroke due to a presumed paradoxical embolism from a PFO are usually young and otherwise healthy individuals, which makes preventing recurrent strokes and long-term disability even more important.
This new FDA approval allows such patients, when clinically indicated, to have access to this preventive therapy. However, caution should be used when assessing the need for PFO closure in patients with a cryptogenic stroke, as the prevalence of PFO in the general population is high (25%–30%).1 A PFO identified during a stroke workup may be incidental and not associated with the index stroke event. Closure of a PFO in this setting would not impart any clinical benefit and would expose the patient to the known risks of PFO closure. PFO closure should only be performed with shared decision making after a careful multidisciplinary evaluation to ensure optimal patient outcomes and patient-centered care.
FDA Approval Process
The approval process of a PFO closure device in the United States has been a long and challenging journey (Figure 1). Prior to October 31, 2006, PFO closure was performed under HDE use for patients who had a recurrent cryptogenic stroke from a PFO and had failed conventional medical therapy. Devices that had received HDE approval were the Amplatzer PFO occluder and CardioSeal (NMT Medical, Inc.). In 2006, the HDE approvals for these devices were voluntarily withdrawn, as the number of patients eligible exceeded the regulatory limit for the use of 4,000 patients per year. The three devices that have been studied in the United States in large randomized clinical trials are the Amplatzer PFO occluder, the StarFlex septal occluder (NMT Medical, Inc.), and the Helex/Cardioform septal occluder (Gore & Associates). The Amplatzer PFO occluder is currently the only FDA-approved device for the prevention of recurrent cryptogenic stroke in the United States.

Figure 1. Timeline showing important dates of PFO closure approval trials and FDA milestones in the United States. Currently, the Amplatzer PFO occluder is the only FDA-approved PFO closure device in the United States.
The landmark Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial compared the Amplatzer PFO occluder to medical therapy for the prevention of stroke recurrence in 980 patients.2 The initial results were presented at the 2012 Transcatheter Cardiovascular Therapeutics (TCT) scientific sessions. The intention-to-treat primary analysis demonstrated a nonstatistically significant reduction in recurrent stroke for the closure group (mean follow-up, 2.6 years). The as-treated analysis, however, reported a significantly lower rate of recurrent stroke in the closure group as compared to the medical therapy group. The prevalence of atrial fibrillation in both groups was similar. These data were submitted for premarket approval of the device, but the FDA requested additional information, including supplementary analysis. Over the following 3 years, attempts to satisfy the FDA’s requests were made, and a proposal for limited use was submitted, but no agreement was reached.
In October 2015, investigators from the RESPECT trial presented long-term follow-up data at TCT.3 After an extended mean follow-up of 5.9 years for these patients, the intention-to-treat primary analysis demonstrated a significant reduction in recurrent ischemic strokes in the PFO closure arm (hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.305–0.999; P = .046). The reduction in new strokes of unknown mechanism, excluding recurrent events from a known ischemic cause, was even more significant in the closure arm (HR, 0.38; 95% CI, 0.18–0.79; P = .007). Device embolization or erosion was not observed, and atrial fibrillation rates were similar in both groups. There was, however, an unexplained higher rate of deep venous thrombosis and pulmonary embolism in the closure group. Possible explanations include an increased risk for venous thrombosis in PFO patients and greater use of warfarin therapy in the medical group. These new results were submitted to the FDA.
In May 2016, an FDA advisory panel reviewed the complete data and voted 15 “yes” to 1 “no” for device safety, 9 to 7 for effectiveness, and 11 to 5 for benefits of the device outweighing the risks, respectively.4 As a result, on October 28, 2016, the FDA finally approved the Amplatzer PFO occluder for “percutaneous transcatheter closure of a PFO to reduce the risk of recurrent ischemic stroke in patients, predominantly between the ages of 18 and 60 years, who have had a cryptogenic stroke due to a presumed paradoxical embolism, as determined by a neurologist and cardiologist after an evaluation to exclude known causes of ischemic stroke.”
The FDA also required a postapproval study to further assess the long-term safety and efficacy of the Amplatzer PFO occluder. The primary effectiveness endpoint of this single-arm, multicenter, postapproval study is recurrent stroke at 5 years and will include approximately 1,200 patients. The primary safety endpoint is the cumulative incidence of device- or procedure-related serious adverse events at 30 days, including atrial fibrillation, deep venous thrombosis, and pulmonary embolism after PFO closure. This postapproval study will also evaluate the effectiveness of the mandated training programs for new operators, which we discuss further in the training requirements section. The final results of the long-term follow-up of the RESPECT trial were finally published in September 2017.5
Other randomized clinical trials for PFO closure
Two previous randomized clinical trials have failed to demonstrate a benefit for PFO closure over medical therapy. The CLOSURE I trial randomized 909 patients with cryptogenic stroke to PFO closure using the StarFlex septal occluder or best medical therapy in the United States and Canada.6 The StarFlex septal occluder was not superior to medical therapy in preventing recurrent stroke or early death at 2 years. In addition, nearly half of the strokes in the device arm occurred within the first 30 days, suggesting that they could have been related to the device placement. The device arm also experienced a 5.7% risk of atrial fibrillation after the procedure, which could have increased the incidence of stroke in that group. The second trial was the Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism (PC) trial, which compared PFO closure with the Amplatzer PFO occluder to medical therapy in 414 patients for the prevention of death, stroke, transient ischemic attack, and peripheral embolism.7 The trial enrolled patients in Europe, Canada, Brazil, and Australia. Despite a longer mean follow-up of 4.1 years, the PC trial reported similar outcomes in both groups of patients.
On May 16, 2017, the results of the REDUCE and CLOSE clinical trials were presented at the European Stroke Organization Conference. The final results of both trials were published in September 2017 in the New England Journal of Medicine.8,9 The REDUCE trial randomized 664 patients with a previous embolic cryptogenic stroke to PFO closure using Gore & Associates’ septal occluder devices or antiplatelet therapy. The majority (81%) had a moderate-large interatrial shunt. PFO closure was found to be significantly superior to medical therapy at 3.2 years, with a 76.6% relative risk reduction in recurrent clinical stroke (HR, 0.23; 95% CI, 0.09–0.62; P = .002). The trial also demonstrated a reduction in the incidence of new brain infarcts in the PFO closure group (5.7% vs 11.3%; relative risk, 0.51; 95% CI, 0.29-0.91; P = .04). The incidence of new silent infarcts was similar in both groups (P = .97); however, there were significantly higher rates of atrial fibrillation reported in the device closure arm (6.6%), which was mostly periprocedural and transient.8 The data of the Cardioform Septal Occluder device for PFO closure to prevent recurrent stroke has been submitted to the FDA for review. A decision is expected in early 2018. The device is currently approved in the United States for closure of atrial septal defects.
The CLOSE trial is a French study that enrolled patients who suffered a recent cryptogenic stroke and had a PFO with either an atrial septal aneurysm or a large shunt. A total of 663 patients were randomized into three groups: (1) PFO closure using one of the CE Mark–approved PFO devices in addition to antiplatelet therapy, (2) chronic oral anticoagulation, or (3) chronic antiplatelet therapy. During a mean follow-up of 5.3 years, PFO closure with antiplatelet therapy was found to be superior to antiplatelet therapy alone at reducing the risk of recurrent stroke, with an absolute risk reduction of 4.9% (HR, 0.03; 95% CI, 0–0.26; P < .001). There was no stroke reported in the PFO closure group. Oral anticoagulation and antiplatelet therapy did not differ. Again, the device closure arm had a significantly higher incidence of atrial fibrillation (4.6%).9

Patient Selection
Presently, PFO closure is approved for patients who have a documented cryptogenic stroke from a presumed paradoxical embolism. Stroke symptoms should last ≥ 24 hours; if they last < 24 hours, they must be associated with a new, neuroanatomically relevant cerebral infarct on noninvasive imaging. Evidence supporting a paradoxical embolism includes cortical infarcts in multiple vascular distributions and infarcts of different ages in the same territory.10 Lacunar infarcts are not usually associated with embolic events.11 Other supporting elements include the absence of conventional stroke risk factors, a history of a deep vein thrombosis or pulmonary embolism before the stroke, recent travel, and Valsalva maneuver preceding the event.12
The Risk of Paradoxical Embolism (RoPE) score is a useful tool to assess the likelihood that the PFO is responsible for the event stroke (Table 1).13 A high score suggests that the stroke is most likely related to the PFO. Conversely, a low RoPE score is associated with more recurrent embolic events than a higher RoPE score, as shown in Figure 2.13 This can be explained by the fact that the rate of recurrent PFO-related cryptogenic stroke is lower (1.6% per year)14 when compared to other mechanisms of ischemic stroke (8%–12% per year).15
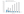
Figure 2. The fraction of stroke attributed to a PFO and the recurrence rate of stroke/TIA at 2 years are presented for a RoPE score value ranging from 0 to 10. As the RoPE score increases, the fraction of stroke attributable to a PFO goes up, but the recurrence rate decreases.
Anatomical predictors of recurrent embolic events are not currently well established. Although the prevalence of a PFO is five times higher in cryptogenic stroke than in nonstroke patients, the presence of a PFO and the size of the right-left shunt have not been clearly linked to an increased risk of stroke.16 The combination of a PFO and an atrial septal aneurysm, however, have been shown to be a significant risk factor for recurrent stroke and, when present, support PFO closure.17 The Chiari network or Eustachian valve have also been associated with an increased risk of clot formation and right-left shunting.18
Patients with suspected cryptogenic stroke should be fully evaluated by a neurologist to confirm the diagnosis and exclude other causes of ischemic stroke before PFO closure. A suggested workup for exclusion of other causes is presented in Table 2.9,19,20 One of the most important conditions to exclude is atrial fibrillation. Unmasking occult atrial fibrillation or atrial flutter in patients with unexplained stroke would suggest an association and mandate guideline-directed chronic anticoagulation. Such patients would generally not be considered for percutaneous PFO closure. Prolonged monitoring has identified occult atrial fibrillation in up to 16% of cryptogenic stroke patients.21 Hence, continuous cardiac monitoring should be performed for a minimum of 30 days before PFO closure, especially in patients with low RoPE scores. If atrial fibrillation is not detected during that time and a multidisciplinary team believes that the index stroke is PFO related, it is reasonable to consider PFO closure. Patients with intracardiac mass, vegetation, tumor, or thrombus at the intended site of implantation or documented evidence of venous thrombus in the vessels through which access to the PFO will be attempted, should not undergo closure until that condition is resolved.

Heart-Brain Team Evaluation
The benefit of a patient-centered, multidisciplinary team evaluation for structural heart disease procedures has already been demonstrated for transcatheter aortic valve replacement, percutaneous mitral valve repair, and left atrial appendage closure. The same concept has now been introduced for PFO closure, as mandated by the FDA. A heart-brain team fosters a shared decision-making process between the patient, a neurologist, and a cardiologist and is essential to ensure proper patient selection for PFO closure. The FDA approval clearly mandates shared decision making in the patient selection process. This multidisciplinary approach has the benefit of preventing inappropriate PFO closure procedures and exposing patients to unnecessary risks. In addition, patients who are found to have an abnormal hypercoagulable workup should be referred to a hematologist for further evaluation and workup.
Training Requirements
To ensure optimal outcomes, PFO closure should be performed at high-volume, experienced centers that routinely perform transseptal left-sided cardiac structural interventions. Data from other structural procedures have shown that low-volume centers have inferior patient outcomes.22 Individual operators should also have the cognitive and technical skillsets required to safely perform this procedure with a low complication rate. Therefore, when approving the Amplatzer PFO occluder, the FDA mandated the implementation of a rigorous physician training program. Every physician, regardless of previous experience, must undergo specific didactic training and case support for the first cases. The Society for Cardiovascular Angiography and Interventions task force survey for structural heart disease interventions previously established that a minimum of 15 PFO closures were required for operators to achieve proficient skills.23 The FDA has set a requirement of 25 implantation procedures for certification.
The FDA mandates that experienced operators, defined as having previously performed 25 or more septal closure procedures using the Amplatzer device, receive proctoring for the first cases by certified personnel from St. Jude Medical, Inc. That proctor will assess whether the physician is ready to perform the procedure safely and independently. For operators with < 25 previous implantations, the first cases will be proctored by a certified physician proctor until that physician can certify that the operator is ready to perform cases without supervision.
The ideal training for PFO closure for new operators remains embedded in a dedicated structural heart disease intervention fellowship. Other training opportunities available include didactics focused on basic principles of PFO occlusion, hands-on experience with device-specific equipment, simulation devices, and viewing live cases performed by experienced physicians in an interactive format.
Third-Party Payer Insurance Coverage
Before FDA approval, many if not most third-party payers had a noncoverage policy for percutaneous PFO closure procedures. However, after the FDA approval, private United States payers have started to update their coverage policy for PFO closure after a cryptogenic stroke. To optimize the prospect of gaining coverage by private payers, prior authorization submission should be accompanied by a clinical encounter that contains all of the following: (1) a description of the cardiac imaging confirming the presence of the PFO, (2) a description of the cerebral imaging identifying a stroke(s) and mentioning the likelihood that it was embolic in nature, (3) a notation that the patient was evaluated by a neurologist and a cardiologist and that both agree that PFO closure is reasonable to prevent a recurrent event, (4) a calculated RoPE score, (5) the results of 30-day cardiac event monitoring documenting an absence of atrial fibrillation/atrial flutter, and (6) a description of the tests performed to exclude other causes of ischemic stroke.
Although there is no specific diagnostic code for cryptogenic stroke in the current International Classification of Diseases, I63.9 (cerebral infarction, unspecified) can be used as a general code for the diagnosis. The current procedural terminology code that should be used for percutaneous PFO closure is 93580 (percutaneous transcatheter closure of congenital interatrial communication [ie, Fontan fenestration, atrial septal defect] with the use of an implant). Operators should keep in mind that, currently, the Amplatzer PFO occluder is the only device approved in the United States for this application.
Conclusion
The journey leading to the approval of a dedicated device for percutaneous closure of PFO in the United States has been long and arduous. The availability of the Amplatzer PFO occluder fulfills an important unmet clinical need and expands the procedural armamentarium of the structural interventionist. Three large randomized clinical trials have now demonstrated the benefit of PFO closure over medical therapy in preventing recurrent ischemic cryptogenic stroke. It is anticipated that additional clinical trial data will help in further identifying specific patient subsets who will benefit most from PFO closure, such as those with a large shunt or an atrial septal aneurysm. Updated societal guidelines on stroke prevention are now recommending PFO closure for PFO-mediated stroke in patients aged 60 years or younger (level of evidence: A).24 It is our responsibility as proceduralists to provide for the safe and effective delivery of this therapy to the population. To accomplish this, it is essential that we engage in shared decision making with neurologists to ensure adherence to the overarching principle of patient-centered care.
1. Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20.
2. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
3. Carroll JD. RESPECT: a prospective randomized trial of PFO closure in patients with cryptogenic stroke—long-term results. Presented at: TCT 2015; October 15, 2015; San Francisco, California.
4. Rosenfield K, Kavinsky C. Potential victory for patients in FDA advisory panel recommendations on PFO occluder. The Society for Cardiovascular Angiography and Interventions. www.scai.org/Presidents.aspx?cid=ce73b410-90ef-4bfa-9305-e684d441866f#.WPDYfFMrLJw. Published May 26, 2016. Accessed March 17, 2017.
5. Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022-1032.
6. Furlan AJ, Reisman M, Massaro J, et al. Study design of the CLOSURE I trial: a prospective, multicenter, randomized, controlled trial to evaluate the safety and efficacy of the StarFlex septal closure system versus best medical therapy in patients with stroke or transient ischemic attack due to presumed paradoxical embolism through a patent foramen ovale. Stroke. 2010;41:2872-2883.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033-1042.
9. Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011-1021.
10. Dhakal P, Verma V, Bhatt VR. Cryptogenic stroke. N Engl J Med. 2016;375:e26.
11. Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005;76:617-619.
12. Ozdemir AO, Tamayo A, Munoz C, et al. Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism. J Neurol Sci. 2008;275:121-127.
13. Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619-625.
14. Almekhlafi MA, Wilton SB, Rabi DM, et al. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology. 2009;73:89-97.
15. Elkind MS. Outcomes after stroke: risk of recurrent ischemic stroke and other events. Am J Med. 2009;122(4 suppl 2):S7-S13.
16. Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172-1179.
17. Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740-1746.
18. Ning M, Lo EH, Ning PC, et al. The brain’s heart - therapeutic opportunities for patent foramen ovale (PFO) and neurovascular disease. Pharmacol Ther. 2013;139:111-123.
19. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
20. Kiernan TJ, Yan BP, Cubeddu RJ, et al. May-Thurner syndrome in patients with cryptogenic stroke and patent foramen ovale: an important clinical association. Stroke. 2009;40:1502-1504.
21. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
22. Carroll JD. Relationship between procedure volume and outcome for transcatheter aortic valve replacement in U.S. clinical practice: insights from the STS-ACC TVT registry. Presented at: ACC 2016; April 3, 2016; Chicago, Illinois.
23. Marmagkiolis K, Hakeem A, Cilingiroglu M, et al. The Society for Cardiovascular Angiography and Interventions Structural Heart Disease Early Career Task Force survey results: endorsed by the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;80:706-711.
24. Wein T,Lindsay MP, Cóté R, etal. Canadian stroke best practice recommendations: secondary prevention of stroke, 6th edition practice guidelines, update 2017 (epub ahead of print). Int J Stroke. 2017;Jan1.
Marie-France Poulin, MD
Division of Cardiology
Department of Medicine
Rush University Medical Center
Chicago, IL
Disclosures: None.
Clifford J. Kavinsky, MD, PhD
Division of Cardiology
Department of Medicine
Rush University Medical Center
Chicago, IL
Disclosures: None.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!



