Acute Ischemic Stroke in the Third Trimester of Pregnancy: A Case of Successful Treatment with Tenecteplase and Thrombectomy
The authors present a case of ischemic stroke in an individual in the third trimester of pregnancy, with favorable outcomes for both the mother and infant resulting from treatment with both tenecteplase and thrombectomy.
Stroke in pregnancy has an incidence rate of 30 per 100,000, which is ~3 times higher than in similarly aged nonpregnant women.1 Whereas the overall incidence rate remains low, pregnancy and the postpartum period represent substantial risk factors for thrombotic events in younger individuals. Pregnant individuals historically have been excluded from randomized controlled trials of thrombolytic agents, and pregnancy was only recently removed as a contraindication for use of thrombolytic agents in the 2018 American Heart Association stroke guidelines. To date, case reports and retrospective data analysis have demonstrated the safety and efficacy of thrombolysis with tenecteplase (TNKase; Genentech, South San Francisco, CA), alteplase (Activase; Genentech, South San Francisco, CA), and endovascular therapy (EVT) in pregnant individuals.2-9
We report a case of acute ischemic stroke during pregnancy secondary to internal carotid artery dissection and thrombosis treated with tenecteplase and EVT. Previous literature has established the safety and efficacy of combined tenecteplase and thrombolysis for tandem lesions in nonpregnant individuals.10 This is one of the first cases demonstrating the safety and efficacy of combined tenecteplase and EVT in a pregnant individual.
Case Presentation
BL, aged mid-30s, who was pregnant (35 weeks of gestation, G3P2), presented to the emergency department with symptoms of right-sided weakness and unresponsiveness. At ~10:35 that morning, BL had reported symptoms of headache and “not feeling like myself.” BL’s husband noticed that BL was not moving the right side normally and had difficulty answering questions, and called emergency medical services at 10:51 AM. BL arrived at the hospital at 11:58 AM.
BL was evaluated as a code stroke, with an initial National Institutes of Health Stroke Scale (NIHSS) score of 21 because of substantial aphasia and right-sided motor deficits. Tenecteplase was administered at 12:18 PM after a CT head scan revealed no evidence of hemorrhage. CT angiogram and CT perfusion were performed at 12:25 PM, with findings concerning for a left internal carotid artery (ICA) and middle cerebral artery occlusion (Figures 1 and 2). The decision was made to proceed with thrombectomy at 12:40 PM. Throughout the stroke evaluation, an obstetrics/gynecology (OB/GYN) practitioner was present to help guide decision-making and to be present in case of a need for delivery.

Figure 1. CT angiography shows large vessel occlusion.

Figure 2. Perfusion study demonstrates preintervention perfusion deficit.
Diagnostic Process
BL was taken to the endovascular suite, where extensive lead protection was used to limit radiation exposure to the womb. Angiography showed evidence of left cervical to petrous segment ICA dissection, with focal stenosis and occlusion at the midcervical segment and the petrous ICA segment (Figures 3 and 4). The clot at the cervical segment was able to be aspirated, but the neurosurgeon was unable to pass the petrous stenosis despite attempts with multiple catheters and wires. After discussion with the OB/GYN practitioner about the need for dual antiplatelet therapy, the decision was made to proceed with angioplasty and stenting of the ICA. A final angiogram performed after stenting showed an open ICA, but anterior cerebral artery and superior M2 branch occlusion remained (Figures 5 and 6). BL was treated with aspirin and clopidrogel (Plavix; Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Bridgewater, NJ).
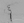
Figure 3. Angiography during thrombectomy demonstrates stent placement.

Figure 4. Angiography during thrombectomy shows tortuosity.
Case Resolution
BL tolerated the procedure well without any complications and was closely monitored in the neurointensive care unit with the OB/GYN team on standby. Complete improvement of weakness occurred but severe expressive aphasia remained. BL was discharged 2 days later after evaluation indicated a NIHSS score of 2.
Cesarean delivery was performed 3 weeks later at 38 weeks of gestation. Clopidogrel was stopped 5 days before the surgery. BL tolerated the delivery without complications and delivered a healthy infant with an APGAR score of 8. Aspirin and clopidogrel were resumed 24 hours after surgery. BL was admitted for observation 16 days after surgery for vaginal bleeding, and ultrasound revealed a 2.8-cm clot in the uterus. The clot passed without incident, and the decision was made to change clopidogrel to Monday and Thursday dosing.
BL had received massage therapy the day before presentation, but otherwise denied any trauma to the neck. BL received outpatient hypercoagulability workup and genetic testing. A heterozygous variant of unknown significance was found in the TNXB gene, which is commonly associated with Ehlers-Danlos syndrome.
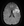
Figure 5. Diffusion-weighted sequence after thrombectomy.

Figure 6. Angiography demonstrates successful reperfusion after thrombectomy.
Discussion
The overall risk of stroke is increased during pregnancy, and stroke represents a substantial source of morbidity in pregnant individuals. The highest risk is in the third trimester and the early postpartum period. More than 50% of pregnancy-related strokes are hemorrhagic, possibly coinciding with hypertensive disorders of pregnancy.11 Carotid dissection shows a similar pattern, with a retrospective study showing approximately doubled rates of spontaneous carotid artery dissection during pregnancy compared with age-matched nonpregnant individuals.12 This increased risk of stroke is thought to be multifactorial, including cardiovascular changes, changes in hormones, changes in vascular compliance, and a prothrombotic state. BL presented in the third trimester with acute ischemic stroke and carotid dissection noted on angiography. Other than pregnancy, risk factors that may have contributed to stroke include recent massage therapy and a variant of unknown significance on a gene commonly associated with Ehlers-Danlos syndrome.
Randomized controlled trials have not been performed in pregnant individuals, but case reports and retrospective studies have reported safety of alteplase in pregnancy, with retrospective studies showing no increases in major systemic bleeding, in-hospital mortality, or adverse events in pregnant vs nonpregnant participants.2-5 A recent case report described administration of tenecteplase to a pregnant individual with a successful outcome for both the mother and the infant.5 Treatment with tenecteplase was chosen due to its improved ease of administration compared with alteplase.5 Both alteplase and tenecteplase are large molecular weight molecules, weighing ~59 kDa, and would not be expected to cross the placenta.13 On March 3, 2025, the FDA approved tenecteplase for the treatment of acute ischemic stroke in adults, making it the first new stroke medication approved in nearly 30 years.
Case reports and retrospective reviews have shown favorable safety and efficacy outcomes of treatment with endovascular mechanical thrombectomy, including in combination with alteplase.6-9 There is a potential concern for thyroid dysfunction with the use of iodine contrast in pregnancy, although studies on the nonionic agents Iopamidol and iopromide (Ultravist; Bayer HealthCare Pharmaceuticals, Whippany, NJ) have shown no teratogenic or mutagenic effects despite crossing the placenta.13 Most iodinated contrasts are classified as pregnancy category B, with the American College of Obstetricians and Gynecologists recommending use in cases when there is a need for diagnostic information that will affect maternal or fetal care and outcome.14 Radiation exposure during the procedure is estimated to be low and well below thresholds for fetal malformation or death.8
Conclusion
Studies on acute stroke interventions in pregnancy, particularly the off-label use of tenecteplase, have been limited. We present an example of intervention with both tenecteplase and thrombectomy, with favorable outcomes for both the mother and infant. Distal occlusions were unable to be removed due to the anatomy of the carotid, but the procedure was well-tolerated. Our case suggests that the combination of tenecteplase and thrombectomy should not be withheld in cases of pregnancy. Further studies on tenecteplase would be beneficial as its frequency of use continues to increase.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Stroke
Blood Pressure Targets After Stroke: How Low Is Too Low?
Hatem Tolba, MD; Filza Sikandar, MBBSHatem Tolba, MD; Filza Sikandar, MBBS



