Care of Individuals with Neurofibromatosis Type 1 Amid an Evolving Paradigm of Therapeutic Development
Treatment for individuals with neurofibromatosis type 1, a complex disease with variabilities in presentation and disease course, requires a multidisciplinary approach with emphasis on surveillance along with ongoing discussion of therapeutic interventions.
Neurofibromatosis type 1 (NF1), previously referred to as von Recklinghausen disease, is the most common hereditary tumor predisposition syndrome, and it can lead to an array of clinical features with primarily neurocutaneous manifestations. This autosomal dominant disorder consists of an inactivating mutation in the NF1 gene on chromosome 17. The NF1 gene encodes for the protein neurofibromin, which acts as a negative regulator of the RAS-MAPK pathway; NF1 is therefore often referred to as a “RAS-opathy” given that tumorigenesis is linked to overactivation of this pathway, leading to increased cell proliferation.1
NF1 has an incidence rate of ~1 in 3000 births worldwide and is a heterogeneous disorder with a wide array of manifestations that can affect multiple organs.2 Neurofibromas are a virtually universal manifestation and are defined as benign peripheral nerve sheath tumors; plexiform neurofibromas (PNs) also develop within peripheral nerves and their nerve sheaths but may also invade and involve local tissue.1
Diagnosis
The diagnostic criteria for NF1 were updated in 2021. In summary, an individual meets diagnostic criteria for NF1 if they have any 2 of the following characteristics: ≥6 café-au-lait macules, axillary or inguinal freckling, either ≥2 neurofibromas OR 1 PN, optic pathway glioma, ≥2 Lisch nodules or choroidal abnormalities, a distinctive osseous lesion (eg, sphenoid dysplasia, tibial bowing), or a heterozygous pathogenic variant in NF1 with variant allele fraction ≥50%2 (Table). Individuals can therefore meet the diagnostic criteria clinically without requiring genetic testing confirmation. Genetic testing may be indicated in certain circumstances, including ambiguity of clinical presentation or preconception genetic diagnosis. Up to 50% of individuals with NF1 do not inherit it from a parent and have a mosaic presentation (ie, they acquired an NF1 variant after fertilization so not all cell lines are affected as opposed to inherited germline mutations, which affect all cells of the body). Individuals with mosaic NF1 have a <50% chance of transmitting NF1 to their offspring, and variant allele fraction is <50%; therefore, their clinical manifestations may be milder.2

Risk of Malignancy and Surveillance Guidelines
There is an increased risk of cancer in NF1 with different prevailing risks depending on age. For example, optic pathway gliomas occur in at least 15% of individuals with NF1. These are usually World Health Organization grade 1 pilocytic astrocytomas that develop during early childhood and are unlikely to arise or progress during adulthood. PNs develop in ~50% of individuals with NF1 and are present in childhood but can grow in the first 2 decades of life. PNs have a ~15% chance of transformation into malignant peripheral nerve sheath tumors (MPNSTs). MPNSTs are nerve-associated sarcomas that are not exclusive to NF1. These are aggressive tumors with substantial associated morbidity and mortality. Therefore, PNs should be monitored closely, and patients are advised to monitor for any symptoms that may point to malignant transformation, including rapid growth, increasing firmness, or pain associated with the lesion.1
There is an increased risk of breast cancer in individuals with NF1. Women with NF1 are recommended to start receiving mammograms or breast MRIs, or both, at age 30 years, rather than the US Preventive Services Task Force recommendation for every-other-year mammography starting at age 40 years in the general population.3 During adulthood, people with NF1 have an increased risk of gastrointestinal stromal tumors, pheochromocytoma, rhabdomyosarcoma, and glomus tumors compared with the general population.1 Updated guidelines regarding the recommended cancer screening for people with genetic cancer predisposition syndromes, including NF1, were published in 2024,3 with surveillance for breast cancer recommended for all individuals with NF1, but additional screening on an as-needed basis for other adult-onset cancers.
Diagnostic evaluation is tailored to the individual, taking age and clinical manifestations into consideration. Individuals with NF1 are recommended to undergo regular comprehensive ophthalmologic examinations, have close follow-up with a dermatologist, and be evaluated for clinical signs of pheochromocytoma with regular blood pressure checks. Other diagnostic testing is obtained on a case-by-case basis; for example, in adults, MRI scans of the brain and orbits are obtained in case of clinical symptoms that could be consistent with a glioma or other structural abnormality, and [¹⁸F]fluorodeoxyglucose positron emission tomography (FDG-PET) scans are obtained to evaluate a worrisome or clinically changing PN concerning for malignant transformation, but neither scan would be required without associated clinical concern (Figures 1, 2).
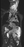

Figure 1. Imaging results demonstrating plexiform neurofibroma. T2 short tau inversion recovery sequence of coronal MRI of the neck, chest, and abdomen demonstrates extensive plexiform neurofibroma infiltration along the left neck and left side of the abdomen, and extending into the pelvis (A). On [¹⁸F]fluorodeoxyglucose positron emission tomography/CT in the same patient, a similar coronal slice shows only mild [¹⁸F]fluorodeoxyglucose avidity (maximum standardized uptake value 3.3) of the plexiform neurofibroma in the neck (B).

Figure 2. Axial postcontrast T1-weighted MRI scan of a right upper-extremity malignant peripheral nerve sheath tumor exhibiting central necrosis, measuring 6.5 cm in the longest diameter.
MPNST Imaging
Imaging surveillance of PN for malignant transformation to MPNST is imperative for early diagnosis. There are characteristics on MRI scans that can be used to distinguish benign peripheral nerve sheath tumors (including neurofibromas and schwannomas) from MPNSTs, although no pathognomonic sign exists. The “target sign” refers to a lesion with a high-intensity signal peripherally and a low-intensity signal centrally on T2-weighted and diffusion-weighted MRI sequences, which corresponds to central collagenous and peripheral myxomatous tissues in neurogenic tumors. The target sign is seen more commonly in benign peripheral nerve sheath tumors due to replacement of these tissues by malignant cells in MPNST, but it has rarely been reported in MPNST.4 MRI characteristics associated with MPNST include larger lesion size, irregular shape, and poorly defined margins.5 FDG-PET imaging is more sensitive but less specific than MRI for detection of MPNST in NF1; out of 99 lesions in 1 study, FDG-PET using a standardized uptake value cutoff of 3.5 had a sensitivity of 100% and specificity of 73.4%, compared with 66.7% and 97.0% for MRI, respectively.6
Treatment Options for NF1 Manifestations
Treatment options have expanded over the past several years. Koselugo (selumetinib; AstraZeneca Pharmaceuticals, Wilmington, DE) was the first medication to be granted Food and Drug Administration approval for the treatment of children with NF1-associated, symptomatic, inoperable PN. Selumetinib is a mitogen-activated protein kinase (MEK) inhibitor of both MEK1 and MEK2, which are key signaling molecules in the activated RAS pathway.1 Approval was given in 2020 after results from the phase 2 AZD6244 Hydrogen Sulfate for Children With Nervous System Tumors (SPRINT) clinical trial (NCT01362803) were published.7
In the SPRINT trial, children with inoperable PNs received selumetinib at a dose of 25 mg/m2 twice daily in 28-day cycles.7 The primary end point was evaluation of objective radiographic response of PNs to selumetinib based on volumetric MRI analysis using the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria.8 Overall, 37 of the 50 participants (74%) exhibited a partial response (defined as a neurofibroma volume decrease of ≥20% from baseline in the target measurable lesion), 34 participants (68%) experienced confirmed partial response (ie, partial responses noted on consecutive MRI scans at least 3 months apart), and 28 participants (56%) demonstrated a durable partial response (ie, partial response on imaging lasting at least 12 cycles). Clinically significant improvement in symptoms and functional status was reported based on questionnaires given to participants and their families, including child-reported pain intensity, clinically meaningful increases in quality-of-life measures, and improvement in motor dysfunction and airway impairment related to the neurofibromas.7 This study followed a phase 1 trial, which had demonstrated similar results in terms of partial response rate measured by imaging, in which 71% of participants (24 of the 30 children) experienced confirmed partial responses using the same measures described in the phase 2 study but differing dose levels.9
A recently published phase 2 clinical trial evaluating the efficacy of selumetinib in adults with inoperable PNs reported that 63.6% of participants (23 of 30) demonstrated partial response on imaging after treatment with selumetinib, using a similar structure as the phase 1 and 2 trials in children, also using the REiNS criteria.10 A phase 3, randomized, double-blind, placebo-controlled trial (NCT04924608) evaluating the safety and efficacy of selumetinib in this population is ongoing (Efficacy and Safety of Selumetinib in Adults with NF1 who have Symptomatic, Inoperable Plexiform Neurofibromas [KOMET]).
On February 11, 2025, the Food and Drug Adminis-tration approved the MEK1/2 inhibitor Gomekli (mirdametinib; SpringWorks Therapeutics, Stamford, CT) for the treatment of both adults and children with NF1 who have symptomatic and unresectable PNs.11 This followed the MEK Inhibitor Mirdametinib (PD-0325901) in Patients With Neurofibromatosis Type 1 Associated Plexiform Neurofibromas (ReNeu) phase 2b clinical trial (NCT03962543) evaluating the efficacy of mirdametinib in the treatment of adults and children with symptomatic NF1-related PNs, which was published in 2024.12 In that study, 24 out of 58 adult participants (41%), and 29 of 56 children (52%) had objective response seen on MRI scans during the studied 24-cycle treatment phase based on ≥20% reduction in the target PN size.
Surgical resection for neurofibromas, especially PNs, poses a challenge given that neurofibromas encase the affected nerves and removal may lead to further nerve injury. Neurosurgeons who specialize in peripheral nerve disorders may be able to debulk a lesion if it is symptomatic, such as a painful PN that is contributing to weakness. In the case of a PN that undergoes malignant transformation into MPNST, complete surgical resection with negative margins is the most effective treatment often followed by local radiation therapy with the goal of decreasing the risk of recurrence. Systemic chemotherapies have not been as successful in the treatment of MPNST as in PN, and surgical excision followed by radiation therapy remains the mainstay of treatment, despite investigation into systemic options.13 MPNST systemic therapy clinical trials thus far have failed to demonstrate a significant clinical benefit for the epidermal growth factor receptor inhibitor erlotinib,14 the vascular endothelial growth factor/platelet-derived growth factor receptor inhibitor sorafenib,15 or a combination of the vascular endothelial growth factor inhibitor bevacizumab and the mammalian target of rapamycin inhibitor RAD001 (Study of Everolimus With Bevacizumab to Treat Refractory Malignant Peripheral Nerve Sheath Tumors [SARC016]; NCT01661283).16
Pediatric to Adult Care Transition
The process of transitioning from pediatric to adult care in the setting of chronic illness has historically been challenging, especially when individuals have had longstanding relationships with their pediatric care providers. Many potential barriers to effective health care transition from pediatric to adult specialty care have been identified, including less access to support or care coordination services in adult clinics, lack of communication about the transition process, discomfort with the sense of losing the caretaker role, and financial and insurance issues. To facilitate the transition from pediatric to adult care, it is recommended that a standardized process be in place, such as using a defined age cutoff that is discussed ahead of time (most commonly 18 years) and handoff between the pediatric care provider and the primary adult care provider who will be assuming the individual’s health care.17 Multidisciplinary care is essential, including ongoing education and support regarding potential complications individuals could face in adulthood.
Conclusion
NF1 is a complex, heterogeneous tumor predisposition syndrome that requires multidisciplinary management, close follow-up, consideration of surveillance imaging, and up-to-date knowledge of the ever-changing landscape of treatment options—both available and in development—for individuals with NF1. Work to improve outcomes among individuals with NF1 remains; however, a great deal of progress has been made in the past several years, with more on the horizon.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Neuro-oncology
Modern Glioma Management: Integrating Molecular Markers and New Targeted Therapies
Patrick Y. Wen, MDPatrick Y. Wen, MD



