Neuromyelitis Optica Spectrum Disorder With Atypical Imaging Features
We present an unusual, diagnostically challenging case of neuromyelitis optica spectrum disorder with a trident sign and nerve root enhancement on imaging.
Case Presentation
MA, aged late 20s, presented to the emergency department with 2 weeks of radicular back pain and allodynia in the feet. MA described achy low back pain with episodic electric shock–like sensations beginning in the thighs and traveling distally to the arches of the feet. This progressed to burning pain and ascending paresthesias in both legs, with sensitivity to touch in both feet. MA also endorsed 2 days of increased fecal urgency, but no bowel or bladder incontinence. On review of systems, MA reported a several-year history of episodic, painless, nonpruritic rash on the trunk and legs. MA denied any history of recent falls, trauma, heavy lifting, travel, sick contacts, systemic symptoms, or previous episodes similar to the current presentation. The family history was remarkable for an isolated episode of unilateral sensory symptoms in MA’s mother when she was in her early 20s, which resolved with steroid treatment, but no formal diagnosis was given.
On general examination, vital signs were within normal limits. Hyperpigmented, macular, nonerythematous lesions were noted on the back, left flank, and right leg. On neurologic examination, MA had normal mental status, intact cranial nerves II through XII, and normal bulk, tone, strength, and sensation in both arms. MA had diminished sensation to light touch, temperature, and vibration in the perianal and saddle areas and below the knees, with impaired proprioception in the toes and hyperesthesia in the feet. No sensory level was appreciated. Leg strength appeared to be largely preserved, although formal tone and strength testing were limited by severe radicular pain. Deep tendon reflexes were brisk and symmetric, except for the patellar reflex, which exhibited a brisk response on the left and trace response on the right; toes were downgoing bilaterally. Rectal tone was normal. Gait was wide-based and the Romberg sign was positive.
The presentation of bilateral dysesthesias in the lower extremities, with intact higher cortical, cranial nerve, and upper extremity function, suggested either a thoracic spinal cord lesion or a process involving peripheral nerves or nerve roots of both legs. Prominent low back and radicular pain in conjunction with a diminished patellar reflex on the right were suggestive of lumbosacral nerve root involvement. The presence of saddle anesthesia and fecal urgency raised concern for cauda equina syndrome. The relative preservation of strength and involvement of multiple sensory modalities in multiple dermatomes was suggestive of pathology affecting the sensory tracts of the spinal cord. Overall, a myeloradicular process seemed most likely.
Diagnostic Process
MRI scans of the cervical, thoracic, and lumbar spine with and without contrast revealed an enhancing lesion extending from the distal thoracic cord to the conus medullaris, consistent with longitudinally extensive transverse myelitis (LETM; Figure, A and B). Additional enhancing lesions were present along the dorsal surface of the spinal cord at the T4 level (Figure, B), and at the distal aspect of the thecal sac (Figure, A). There was also faint enhancement of some of the lower thoracic dorsal roots (Figure, C). On the cross-section of the lower thoracic lesion, the trident sign could be readily appreciated (Figure, D). Brain MRI with and without contrast was unremarkable.
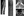
The differential diagnosis for subacute LETM includes autoimmune, infectious, neoplasm, and less likely nutritional, metabolic, or vascular etiologies. The absence of systemic signs or leukocytosis suggested against an infectious process, but a search for an infectious agent in cerebrospinal fluid (CSF) was indicated. Metastatic malignancy is implausible in a young person with no systemic symptoms, and could be more definitively ruled out if results on whole-body CT scanning were normal. Spinal cord infarct did not fit with the subacute time course, the symptoms, or the multifocal nature of lesions on MRI. Spinal dural arteriovenous fistulas could present subacutely, but the imaging was not consistent with this etiology. An autoimmune etiology was favored.
Lumbar puncture revealed a mildly elevated protein level of 48 mg/mL, lymphocytic pleocytosis (44 leukocytes with 93% lymphocytes), 0 erythrocytes, and 19 CSF-restricted oligoclonal bands. Flow cytology showed no evidence of malignancy. CSF cytometry disclosed a mildly elevated CD4:CD8 ratio of 4.8:1, with no B cells on 2 sequential lumbar punctures (Table). Chest, abdomen, and pelvis CT scanning did not show evidence of lymphadenopathy or mass lesions. The presence of a large number of CSF-restricted oligoclonal bands (n=19) was more suggestive of inflammation or infection than stroke or malignancy. An extensive infectious and neoplastic workup returned negative results (Table), and an autoimmune process remained the most likely diagnosis.
The differential diagnosis for autoimmune LETM includes neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody–associated disorder, neurosarcoidosis, and, less commonly, myelitis attributable to systemic lupus erythematosus, Behçet disease, Sjögren disease, or paraneoplastic autoimmune syndromes. Further serum testing revealed a low-positive aquaporin-4–immunoglobulin G antibody (AQP4-IgG) titer of 1:10 on enzyme-linked immunosorbent assay testing.
Case Resolution
Despite the low-positive AQP4-IgG result, certain aspects of the presentation were atypical for NMOSD.1 The presence of CSF-restricted oligoclonal bands is seen in a minority of NMOSD cases.2 Root involvement, as seen in MA (Figure, C), is unusual but has been reported.3,4 Mildly elevated CSF CD4:CD8 and lymphocytic pleocytosis could be suggestive of, but not specific for, neurosarcoidosis.5,6 A history of dermatologic eruptions could represent an unrelated condition, but could also be a manifestation of a systemic inflammatory process, such as sarcoidosis. In addition, postgadolinium T1-weighted axial MRI revealed a pattern of central and dorsal-subpial enhancement, referred to as the trident sign, which has been reported in sarcoid myelitis but not in AQP4-IgG–positive NMOSD (Figure, D).7
AQP4-IgG antibody seropositivity is highly specific for NMOSD, but false positives may occur at low titers, especially when testing is performed using an enzyme-linked immunosorbent assay rather than cell-based assay.8,9 Given MA’s low-positive AQP4-IgG titer and various laboratory and imaging features atypical of NMOSD, testing was repeated using a gold standard cell-based assay, which revealed an unequivocally positive titer of 1:100. Thus, MA met the diagnostic criteria for NMOSD on the basis of the clinical presentation of myelitis, longitudinally extensive lesion on MRI, and anti–AQP4 antibody seropositivity.1 Whole-body [¹⁸F]fluorodeoxyglucose positron emission tomography–CT scanning showed no abnormal uptake to suggest sarcoidosis.6 A skin biopsy of MA’s rash revealed only atopic dermatitis.
MA was treated with high-dose intravenous steroids for 5 days, followed by an extended oral prednisone taper, with improvement of the symptoms. Following serologic confirmation of AQP4-IgG–positive NMOSD, MA was started on rituximab, which has been well-tolerated. At a recent follow-up 11 months after hospitalization, neurologic examination results were within normal limits, and MA was largely symptom-free, with only occasional lower extremity cramping.
Discussion
AQP4-IgG–positive NMOSD is an autoimmune disease of the central nervous system mediated by autoantibodies to the AQP4 water channel (AQP4-IgG), which are located primarily on astrocytes. NMOSD has a mean age at onset in the third to fourth decade of life, and has a strong female preponderance (up to 9:1).9 The course is usually relapsing, and attacks may be severe and result in substantial disability.9 Based on the international consensus diagnostic criteria for NMOSD, diagnosis of AQP4-IgG–seropositive NMOSD requires meeting 1 of 6 core clinical or imaging characteristics of the disease (ie, optic neuritis, acute myelitis, area postrema syndrome, acute brainstem syndrome, acute diencephalic syndrome, or symptomatic cerebral syndrome, with typical lesion pattern on MRI) and the exclusion of alternative diagnoses.1 Isolated LETM occurs as the initial presenting feature in up to one-third of cases of NMOSD.10 Lesions typically have a predilection for the central cervical cord and cervicomedullary junction (where the density of AQP4-IgG is particularly high), and may demonstrate partial ring enhancement on postgadolinium axial T1-weighted sequences.11 Bright spotty lesions, defined as extremely hyperintense lesions on axial or sagittal T2-weighted MRI, have been reported as a specific finding in NMOSD.11 However, bright spotty lesions also can be seen with severe cord injury from other causes, such as spinal stroke or trauma.11
Sarcoidosis is a systemic immune-mediated disorder characterized by the formation of noncaseating granulomas in affected tissues.12 A total of 5% to 10% of people with sarcoidosis present with a neurologic syndrome, with myelopathy accounting for 5% to 25% of these cases.6,13 Diagnosis of neurosarcoidosis is notoriously challenging because of the heterogeneity of clinical presentation. When the spinal cord is involved, enhancement along the cord’s dorsal surface is common, and cauda equina involvement with enhancement along nerve roots may be seen, both of which were present in our case (Figure, A and B).3,11 Spinal leptomeninges can also be affected.11 The trident sign is a radiologic finding proposed to have high specificity for the diagnosis of neurosarcoidosis.7 Several case reports have specifically cited its usefulness for differentiating neurosarcoidosis from NMOSD in cases of atypical imaging findings or false-positive AQP4-IgG antibodies.7,14,15 The trident sign was visualized on the thoracic MRI in MA, whose laboratory investigations and response to treatment, as well as the absence of extraneural involvement or lymphadenopathy on whole-body positron emission tomography–CT scanning, ultimately supported a diagnosis of NMOSD (Figure, C). A recent case report also documented the trident sign in an individual with spinal cord lymphoma, bringing into question the specificity of this radiologic finding for neurosarcoidosis.16 This sign needs to be assessed systematically in people with or without neurosarcoidosis to clarify the sensitivity and specificity of this finding, and to help clarify its immunopathologic basis.
Summary
We present a diagnostically challenging case of NMOSD with a trident sign, which, to our knowledge, has not been previously reported in NMOSD. This case highlights the wide-ranging differential diagnosis for LETM and the importance of synthesizing clinical history, examination findings, imaging results, and laboratory studies to reach a diagnosis.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- MS & Immune Disorders
Radiologic Differential Diagnosis of Multiple Sclerosis
Jared V. Goodman, MD, PhD; Apisit Kaewsanit, MDJared V. Goodman, MD, PhD; Apisit Kaewsanit, MD - MS & Immune Disorders
Radiologic Biomarkers in Multiple Sclerosis: Improving Detection and Diagnosis
Aisha Elfasi, MD; Valeria Fagundo, MDAisha Elfasi, MD; Valeria Fagundo, MD - MS & Immune Disorders
Kappa Free Light Chain Index in the Diagnosis of Multiple Sclerosis
Jeffrey Dunn, MDJeffrey Dunn, MD



