CADASIL Misdiagnosed as Multiple Sclerosis
The authors present a case of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy misdiagnosed as multiple sclerosis.
Case Presentation
SN, aged mid-40 years, who had left-sided tingling and numbness, mild right hemiparesis, and left-sided Duane syndrome—a congenital eye movement disorder characterized by the inability to abduct the affected eye while maintaining normal or limited adduction—presented for a genetic evaluation. Medical history was significant for hypertension, hyperlipidemia, and multiple sclerosis (MS), diagnosed 6 years before the genetic evaluation and treated with ocrelizumab (Ocrevus; Genentech, South San Francisco, CA) for 2 years. Three years after the MS diagnosis, SN had an ischemic stroke presenting with a 2-day history of right leg weakness and slurred speech. MRI demonstrated acute infarction in the right cerebral hemisphere and posterior subinsular region extending to the left corona radiata. SN was managed with aspirin and high-intensity statin therapy, along with physical and occupational therapy, resulting in residual mild right-sided hemiparesis.
The Review of Systems revealed chronic back pain due to manual labor and frequent falls. SN was recognized early in life to have Duane syndrome but had no other birth defects. SN met childhood developmental milestones without delay but did not complete high school. SN had a history of former smoking (1 pack daily for 34 years); a 20-year history of alcohol use; and a history of frequent cocaine use beginning at age mid-20 years, which tapered to occasional use before ceasing 4 or 5 years before presentation.
A 4-generation family history (Figure 1) revealed 2 siblings with MS. One sibling, TN, who had a history of hypertension and prediabetes, had been originally diagnosed with MS at age 36 years after presenting with episodic headaches and left-sided numbness along with a long-standing right foot drop. TN had reported 3 or 4 previous migraines, as well as several episodes of transient neurologic symptoms characterized by a visual aura of flashing lights, followed by blurred vision in both eyes and numbness of the left side and face. These symptoms typically resolved within 24 hours, except for 1 episode that resulted in permanent dysarthria.
SN’s young child (aged 13 years) had been diagnosed with Tourette syndrome, and a niece (aged 19 years) had a history of seizures. Duane syndrome was present in SN’s older siblings, mother, and maternal grandfather. No family history of stroke was reported.

Figure 1. A 4-generation pedigree obtained at the time of diagnosis. The proband and 2 siblings had been diagnosed with multiple sclerosis. Duane syndrome was present in the maternal grandfather, mother, and younger sister.
Diagnostic Process
Cranial nerve assessment findings were abnormal for cranial nerves II, III, and VI. Loss of visual acuity and lateral rectus weakness were present in the left eye. Medial rectus weakness was found in the right eye. A sensory examination revealed decreased pinprick sensation in the right fingers, ankles, and left toes, as well as allodynia in the right foot. Finger-to-nose and heel-to-shin coordination testing showed impairment on the right side. Deep tendon reflexes were brisk (3+) with right-sided Hoffman sign, upgoing toes, and moderate bilateral ankle clonus. SN was unable to walk in a tandem gait and had a right paretic gait.
A review of an initial brain MRI scan, performed 6 years previously at age 40 during the first workup for sudden-onset left-sided numbness with negative stroke evaluation results, revealed moderate patchy foci of increased signal predominantly within the supratentorial white matter, with numerous lesions in the frontal-parietal region without contrast enhancement (Figure 2A). Subsequent MRI scans of the cervical and thoracic spinal cord, performed 1 month after the initial MRI, revealed no demyelinating lesions. Annual brain MRI did not reveal new T2 white matter lesions until 2 years after the initial MRI scan, at age 42 years, when MRI demonstrated progression of white matter hyperintensities in the periventricular, subcortical, and brainstem regions (Figure 2B). At the time, these were interpreted to be indicative of MS, which led to the initiation of disease-modifying therapy with ocrelizumab. Lumbar puncture demonstrated normal cell count, protein level, and glucose level, and was negative for oligoclonal bands.
After ~2 years of ocrelizumab treatment, a reevaluation of previous MRI scans by a different neurologist revealed white matter lesions inconsistent with MS. Notably, sagittal views of the MRI scan taken at age 42 years revealed that the lesions were not abutting the ventricle—a characteristic more consistent with small vessel ischemic changes than MS (Figure 2C).1,2 Additional imaging at the time showed chronic lacunar infarctions in the brainstem and an abnormality in the right anterior temporal lobe (Figure 2D). This raised clinical suspicion of leukoencephalopathy. SN was referred to genetics evaluation for further assessment.
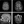
Figure 2. Brain MRI scans of the proband. MRI scan at age 40 years was notable for subcortical white matter hyperintensities (white arrows; A). No contrast-enhancing lesions were noted (A). Progression of subcortical white matter intensity (white arrows) was present at age 42 years (B). Sagittal view of MRI scan taken at age 42 years showed white matter hyperintensities not truly abutting the ventricle (white arrows) (C). MRI scan at age 42 years showed chronic lacunar infarctions in the brainstem (yellow arrows) and an abnormality in the right anterior temporal lobe (white arrow) (D).
Case Resolution
Genetic workup identified pathogenic variants in POLR3A and TRAPPC9, which conferred carrier status for autosomal recessive conditions but would not explain SN’s symptoms. A variant of unknown significance was identified in SOX2 (c.842C>G; p.Ala281Gly; ClinVar ID: 2814306), which was not identified in TN. Follow-up genetic testing revealed a NOTCH3 pathogenic variant in exon 18 (c.2953C>T; p.Arg985Cys; ClinVar ID: 447819). This missense change has been observed in individuals with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). SN began taking 81 mg aspirin daily, has been regularly followed with physical rehabilitation therapy, and has been doing well overall with no further ischemic events.
Further exploration into SN’s family history revealed additional insights, particularly concerning TN, who had a clinical trajectory similar to that experienced by SN. Genetic testing in TN revealed the same pathogenic variant found in exon 18 of NOTCH3 (c.2953C>T; p.Arg985Cys) that was found in SN. In the months following the correct diagnosis of CADASIL, TN began experiencing 30-second episodes of acute severe aphasia. Imaging studies including apparent diffusion coefficient and diffusion-weighted MRI sequences demonstrated an acute stroke in the left precentral gyrus (Figure 3A and 3B). Fluid-attenuated inversion recovery MRI demonstrated substantial small vessel ischemic changes (Figure 3C) and anterior temporal lobe hyperintensities characteristic of CADASIL (Figure 3D).1

Figure 3. Brain MRI scans of the proband’s sibling. Apparent diffusion coefficient sequence demonstrated acute stroke in the left precentral gyrus (yellow arrow) (A). Diffusion-weighted imaging sequence demonstrated acute stroke in the left precentral gyrus (yellow arrow) (B). Fluid-attenuated inversion recovery MRI scan demonstrated substantial small vessel ischemic findings (white arrows) (C), as well as anterior temporal lobe hyperintensities (white arrows)—the characteristic finding of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (D).
Discussion
CADASIL, which has a prevalence of only 2 to 5 in 100,000, as well as symptoms that mimic those of other diagnoses, can be challenging to diagnose.3 CADASIL is characterized by ischemic strokes, vascular dementia, and migraines. However, there is heterogeneity in the clinical presentation. The disease course can also present similarly to MS, with its ability to manifest as a recurring focal neurologic impairment with some recovery between episodes.
SN had evidence of 2 episodes of relapses with new lesions that were disseminated in time, which could have met the 2017 McDonald criteria for an MS diagnosis.1,2 However, factors complicating the case included a history of hypertension, hyperlipidemia, tobacco use, and cocaine use, initially suggesting that the neurologic events were attributable to typical cardiovascular risk factors. In addition, although MS is known to have a modest familial recurrence risk, it was unlikely that multiple members in SN’s family would independently develop MS. A study of the Swedish Multiple Sclerosis Registry involving 28,396 individuals estimated a 2.46% crude risk between siblings.4 Another study from 2023 analyzing individuals from the same registry found that 51% of the variance in MS can be attributed to heritability, with 48% due to nonshared environmental effects and only 1% from shared environmental effects.5 With that in mind, other heritable disorders should be considered in a person with a family history of MS.
The classic findings of CADASIL are bilateral white matter lesions on T2-weighted fluid-attenuated inversion recovery MRI scans involving the periventricular lesions and deep white matter lesions within the frontal, parietal, and anterior temporal regions.6 In MS, these periventricular lesions are ovoid and are in direct contact with the ventricles without intervening white matter, often forming perpendicular to the lateral ventricles, a pattern not commonly seen in CADASIL. In addition, nearly 80% of individuals with MS will develop spinal cord lesions at some point during the disease course.7 Notably, spinal cord lesions were not observed in SN, which further differentiates this case from the typical imaging features seen in MS. Despite these distinguishing features, there are rare instances in the literature where the radiologic presentation of CADASIL has been confounded with MS.8 Such cases highlight the importance of careful analysis and consideration of clinical context in making a differential diagnosis. Radiologic findings demonstrated lacunar infarcts and right anterior temporal lobe hyperintensities in SN (Figure 2D), bilateral anterior temporal lobe hyperintensities in TN (Figure 3D), and small vessel ischemic changes that were more consistent with CADASIL in TN (Figure 3C).6
In SN, a missense variation of arginine to cysteine was found in epidermal growth factor–like repeat 25 of NOTCH3. This is typical for pathogenic variants causing CADASIL.9-12 Exons 2 through 24 of NOTCH3 are known to encode 34 epidermal growth factor-like repeat domains which stabilize the extracellular domain. It is hypothesized that cysteine variants alter the number of disulfide bonds, thus causing a misfolding and a proaggregatory state.13 The presence of NOTCH3-containing granular osmiophilic material in vascular smooth muscle cells serves as a distinctive pathologic hallmark of CADASIL. Despite occasional radiologic and clinical similarities between CADASIL and MS, NOTCH3 sequence variations have not been shown to be associated with MS. A study of 745 simplex families with MS with genotyping on exons 3 and 4 of NOTCH3 failed to show significant results on transmission disequilibrium testing.14
Duane syndrome was present in multiple family members as well as in the proband. However, there have been no published reports describing an association between Duane syndrome and CADASIL. Duane syndrome is generally sporadic; however, 10% of cases are familial. There are at least 3 genes implicated in Duane syndrome—CHN1, MAFB, and SALL4—located on chromosomes 2, 20, and 20, respectively.15 Because NOTCH3 is located on chromosome 19, it is unlikely that NOTCH3 and one of the known Duane syndrome genes are cosegregating. More than 80% of Duane syndrome cases are unexplained by these 3 genes, so it seems more likely that there is an unidentified gene for Duane syndrome near NOTCH3 on chromosome 19 rather than Duane syndrome being a previously unrecognized feature of CADASIL. In a case report of an individual with Duane syndrome and a de novo partial duplication of chromosome 19, the duplication involved 19q14.32; NOTCH3 is at 19p13.12.16 There are >80 Mb between these loci (>80 cM), making cosegregation unlikely.
These cases highlight the complexity of and challenges in diagnosing neurologic conditions with overlapping clinical features. Both SN and TN were initially suspected to have MS, but ultimately diagnosed with CADASIL on the basis of genetic testing. Coexisting conditions and atypical manifestations further complicated the diagnostic process. Awareness of such cases is essential to ensure accurate diagnoses and appropriate management strategies for individuals with similar presentations.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- MS & Immune Disorders
Clinical Neuroinflammatory Mimics of Multiple Sclerosis
Derrick Robertson, MD; Abigail Demers, BSDerrick Robertson, MD; Abigail Demers, BS - MS & Immune Disorders
Updates to the McDonald Diagnostic Criteria for Multiple Sclerosis
Kelsey Stefan, MD; Leena Wahajat, BSKelsey Stefan, MD; Leena Wahajat, BS - MS & Immune Disorders
Kappa Free Light Chain Index in the Diagnosis of Multiple Sclerosis
Jeffrey Dunn, MDJeffrey Dunn, MD



