Neuromodulation Strategies for Lennox-Gastaut Syndrome: A New Frontier
The evolution of neuromodulation strategies for the management of Lennox- Gastaut syndrome emphasizes the role of targeted thalamic stimulation, the importance of individualized approaches, and the need for further research to optimize treatment efficacy.
Lennox-Gastaut syndrome (LGS) is a severe developmental epileptic encephalopathy diagnosed during childhood and continuing through adolescence and adulthood.1 LGS is defined by the International League Against Epilepsy on the basis of clinical and electrographic signs including multiple seizure types that are resistant to drug therapy (in particular tonic seizures in sleep), cognitive and behavioral impairments (which may not be present at seizure onset), and EEG findings of diffuse slow spike-wave and generalized paroxysmal fast activity.2
People with LGS most often meet criteria for diagnosis early in life, from 18 months to 8 years (peak, 3 to 5 years),2 and represent ~1% to 2% of individuals with epilepsy3 who develop drug-resistant epilepsy (DRE). First-line treatment for LGS is antiseizure medication. There are 8 Food and Drug Administration (FDA)–approved antiseizure drugs for treating LGS.4 Most patients with LGS need polytherapy with an average of 3 or 4 medications used. Given the limited medication choices and drug–drug interactions of these medications many individuals with LGS fail to achieve seizure control.
In neuromodulation, nerve activity is altered through targeted delivery of a stimulus—such as electrical stimulation or chemical agents—to specific neurologic sites in the body.5 Neuromodulation is a promising and expanding option for individuals with generalized or multifocal epilepsy who were previously not considered to be surgical candidates. Unlike pharmacologic treatments, neuromodulation therapy exerts continuous effects and is not subject to potential drug–drug interactions. Recent research has further solidified the theory of epilepsy as a network disorder and led to the growing implementation of targeted neuromodulation to specific networks in the management of DRE.
Neuromodulation Devices
Neuromodulation devices surgically implanted for the treatment of DRE act through vagus nerve stimulation (VNS), deep brain stimulation (DBS), or responsive neurostimulation (RNS) (Table 1).
VNS, a well-established open-loop (nonfeedback) system, was approved by the FDA in 1997 to treat DRE and further expanded for pediatric cases (for individuals ≥4 years) in 2017. With VNS, a stimulator is implanted into the left chest with a single lead attached to the vagus nerve. VNS delivers scheduled stimulation regardless of brain activity, which can be effective in reducing seizure burden by 50% to 80% by 2 years in at least 50% to 60% of individuals with LGS.6-8 Unique features include heart rate–responsive autostimulation, manual magnet-activated stimulation, and day/night programming. Adverse effects are primarily dysphonia, hoarseness, neck pain, or cough.8 A low infection rate of 1% has been reported.9

DBS, like VNS, is primarily an open-loop system but is targeted intracranially to stimulate specific subcortical nuclei. DBS was approved by the FDA for adults (aged ≥18 years) in 2018 for the treatment of focal epilepsy via stimulation of the anterior nucleus of the thalamus (ANT). A generator is implanted in the chest, and electrodes are routed intracranially. DBS has been shown to result in 50% to 60% seizure reduction in 50% to 69% of people with LGS.10,11 Unique features include options for cycling or continuous stimulation, frequency band tracking, segmented contacts for directional stimulation, remote programming, and a rechargeable battery with a >15-year battery life. The most frequently reported adverse effects are pain at the implant site, paresthesia, and the potential for depression when targeted in the ANT. The risk of infection varies from 3% to 12%, and the risk of hemorrhage is 3%.9,12
RNS is a closed-loop (feedback) control system. RNS—approved by the FDA in 2013 for adults (aged ≥18 years) with focal DRE—initiates electrocorticography (ECoG) monitoring from electrode contacts at a target site and provides stimulation based on the selected ictal electrographic pattern associated with seizures in up to 2 regions concurrently. The generator is placed into the skull surface, and depth or strip electrodes are then routed intracranially to desired targets. For people with LGS, bilateral centromedian targets are typical; corticothalamic targeting also has been used. RNS has been shown to reduce seizure burden by 50% to 74% in 50% to 70% of individuals with LGS.13,14 Unique features are the closed-loop design, the ability to visualize and program stimulation based on ECoG results, and a cloud-based portal where providers can review uploaded data. Adverse effects include paresthesias, implant site pain, an infection risk of 3% to 13%, and a hemorrhage risk of 5%.9,12
All these modalities have shown increased effectiveness over time without a clear indication of which is most effective.9 Therefore, clinical decisions must be guided by multiple considerations, such as technology accessibility, patient preference, physician and surgeon expertise, and the feasibility of meeting ongoing maintenance requirements.15 A recent survey16 of national pediatric epilepsy centers examining which factors were most important in choosing RNS or DBS for pediatric DRE found the most important factors to be provider preference, patient distance from clinic, and the possibility of future resection. The centromedian nucleus of the thalamus (CMT) was the unanimous target in LGS for RNS and DBS.16
Neuromodulation Evaluation
The process for deciding on neuromodulation treatment and the associated workup can vary between institutions. A general outline of the key steps toward neuromodulation treatment follows (Figure 1).
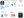
Figure 1. Neuromodulation for Lennox-Gastaut syndrome (LGS): flowchart for evaluation and placement.
Abbreviations: EEG, electroencephalography; LGS, Lennox Gastaut syndrome; sEEG, stereoelectroencephalogram.
Created in BioRender. Edmonds, B. (2025) https://BioRender.com/mz1d6cl
- Phase 1—Evaluation: All people with LGS are considered for presurgical assessment. This typically includes 3T brain MRI with epilepsy protocol sequences, video EEG monitoring, neuropsychologic and genetic testing, and possibly positron emission tomography, functional MRI, or magnetoencephalography.
- Epilepsy Surgery Conference: Results are reviewed in a multidisciplinary meeting (including neurosurgeons, epileptologists, neuroradiologists, and neuropsychologists) to select neuromodulation strategies and targets; if no resectable area or clear seizure onset zone is found, neuromodulation is recommended.
- Neuromodulation Consult and Device Selection: Individuals and families are counseled on device options (VNS, DBS, and RNS), considering technology availability, clinical experience, and maintenance needs. VNS may be preferred as the first device, particularly at some pediatric centers due to its lower FDA-approved age indication (≥4 years) and absence of risk associated with intracranial involvement, but all devices are options. Device features are discussed during consultation with providers to aid selection.
- Neurosurgery Consult: The patient and family meet with a neurosurgeon experienced in functional epilepsy surgery to review the planned surgical procedure.
- Limited Thalamic Stereoelectroencephalography (SEEG) Implant (Optional): In some cases, SEEG is performed before device placement to assess ictal thalamic signal at key nuclei, most often including ANT and CMT, to guide which thalamic nuclei and specific location to target.
- Device Placement and Postoperative Imaging: The device is implanted. Postoperative imaging, typically, CT scan, is performed to confirm location of contacts.
Neuromodulation for LGS: Programming Strategies
Vagus Nerve Stimulation
VNS device programming occurs at the initial postsurgical visit, typically 2 weeks after surgery, though in some cases, the device may be programmed and activated as early as the day following surgery. With the SenTiva M1000 model (LivaNova, London, United Kingdom), the device is programmed to begin the standard autotitration every 2 weeks up to initial goal settings over the course of 16 weeks (Figure 2). For the AspireSR M106 (LivaNova, London, United Kingdom), VNS settings are adjusted at clinic visits every 2 weeks. Initial goal settings for default uptitration based on a large retrospective review of response to treatment among participants in VNS registries are 1.5 mA to 1.75 mA current, 20 Hz to 30 Hz frequency, 250-µs pulse width, ON time 30 seconds, and OFF time 5 minutes.16a

Figure 2. Suggested timeline for device programming.
Abbreviations: DBS, deep brain stimulator; HR, heart rate; PDMS, patient data management system; RNS, responsive neurostimulation; VNS, vagus nerve stimulation.
Created in BioRender. Edmonds, B. (2025) https://BioRender.com/mz1d6cl
Side effects of dysphonia or discomfort with stimulation often improve over time. However, if these side effects persist, adjustment to lower the pulse width, signal frequency, or duty cycle can help. Alternatively, titration can be slowed with 0.125-mA increases, as tolerated. At in-person visits, multiple 0.25-mA increases can be administered within a single visit, followed by observation for 15 to 30 minutes to assess tolerability, allowing the therapeutic range to be achieved more quickly (Table 2).
Deep Brain Stimulation
Two to 4 weeks after DBS surgery, individuals follow-up in clinic for initial programming. The initial setting is typically 1 mA/V bilaterally, and settings are monitored for tolerability in clinic or remotely. Subsequent programming visits typically occur every 3 months. Visits can be in-person or via telemedicine as the individual programmer can be set to allow increases in settings at home with guidance from the treating provider.16b
If an individual with DBS experiences paresthesia, contacts can be evaluated by turning them on and off to identify which contact is causing this side effect. Often the most superficial contact outside of the target nucleus is the culprit. To minimize side effects, clinicians may decrease the pulse width of the stimulation or use segmentation of contacts to direct stimulation away from regions of irritation. If tolerability issues persist or there is a lack of efficacy after optimal programming has been achieved, low-frequency stimulation can be effective in select cases. Programming for the Medtronic (Minneapolis, MN) DBS system can be optimized through the use of BrainSense software, which can capture local field potentials (LFPs) and correlate which LFPs are increased with individual marked seizure events. The response of LFPs to changes in stimulation parameters can be seen in real time in the clinic, which allows for informed patient-specific programming.17

Responsive Neurostimulation
After RNS surgery, individuals follow-up in the neuromodulation clinic at ~4 weeks for initial programming. ECoG results are reviewed before the clinic visit based on regular (ideally daily) data uploads by the patient with seizures marked by magnet swipes. Detectors are programmed based on initial ictal signal for early detection with line length or bandpass. Stimulation is tested in the clinic with active ECoG, and initial thalamic settings are set to 0.5 μC/cm2 for electrodes bilaterally with frequency of 125 Hz, pulse width of 150 μs, and burst duration of 200 to 5000 ms. Follow-up visits are typically every 3 months to review ECoG results, refine detector settings, and adjust charge density or burst duration until optimal seizure reduction is achieved. Charge density for efficacy varies from 0.5 to 3.5 μC/cm2 with typical response rates in the 1.5 to 2.5 μC/cm2 range.
If individuals are experiencing paresthesia or stimulation-related side effects, contacts can be eliminated and tested to evaluate for response. Alternatively, low-frequency (~10 Hz) stimulation settings have been found to help aid tolerability and efficacy for select individuals.18
Target Selection
The thalamus has served as a neurostimulation target since at least 1987 when centromedian nucleus stimulation was used in individuals with LGS, building on the pioneering observations of Wilder Penfield.19 Since then, the theory of epilepsy as a network disease involving central structures has evolved and given rise to targeting of multiple thalamic nuclei including the ANT, CMT, pulvinar, and mediodorsal nuclei.5,20 In people with LGS, there is considerable enhancement of functional connectivity in the thalamus, making this a key target for neuromodulation.21 Most commonly, the centromedian nucleus has been the selected target in LGS because case series have shown efficacy of stimulation at this target and because of the broad connectivity of the centromedian with the frontocentral regions that have notably increased connectivity in LGS. In some cases, the ANT or pulvinar nuclei have been targeted in individuals with LGS but in insufficient numbers to draw conclusions about the efficacy of these targets, and no direct comparative studies evaluating which thalamic nucleus is most efficacious for LGS treatment have been conducted.
Some centers use SEEG data to obtain direct visualization of thalamic activity. This information clarifies whether a clear ictal signature can be seen in the nuclei of interest as well as the characteristics of the region, including frequencies, interictal discharges, and seizure onset pattern. Placement selection of DBS or RNS electrodes is then informed by combining scalp EEG findings, seizure types, and SEEG data indicating which of the individual contacts has a clear ictal signature and earliest thalamic onset.
In an ongoing National Institutes of Health–funded randomized controlled trial (RCT) of RNS (RNS System LGS Feasibility Study), participants with LGS are receiving bilateral corticothalamic implants with each device connected to a depth lead in the centromedian nucleus and a strip lead in the premotor frontal cortex. This approach aims to enable flexible stimulation and detection, offering broader modulation of the LGS epileptic network than thalamic-only stimulation.22
Outcomes and Future Directions
While no head-to-head device trials have been completed, a recent meta-analysis22 of 54 studies comprising 1350 individuals with LGS and neuromodulation compared devices and found a pooled responder rate of 55.4% for all devices (DBS, 69.7%; RNS, 63%; and VNS, 50.6%). For VNS, a recently published LGS-specific dataset from the Registry of Subjects With Drug-Resistant Epilepsy and Treated With the VNS Therapy System (CORE-VNS)8 reported that at 24 months of follow-up, 66.7% of participants with focal seizures and 47.4% with generalized seizures were responders (defined as ≥50% reduction in seizure frequency); 20% of these participants had a ≥80% reduction in total seizure frequency. For RNS, a single-center retrospective study of 10 individuals with LGS revealed a 60% responder rate,13 with other smaller retrospective reviews showing 75% to 100% responder rates.23,24 For DBS, one notable double-blind RCT has been performed including participants with LGS receiving DBS stimulation of the CMT. The Electrical Stimulation of Thalamus for Epilepsy of Lennox-Gastaut phenotype (ESTEL) study evaluated bilateral CMT-DBS in 19 young adults with LGS and showed that 50% had ≥50% reduction in diary-recorded seizures and 89% experienced reduction in electrographic seizures at 6 months of treatment.25
VNS, DBS, and RNS have unique characteristics, and their different benefits may make one option more suitable for a particular individual than the others. VNS can be a good option for individuals who prefer a consistent treatment with little hands-on management required. VNS is a safe and well-tested option for individuals who want to avoid intracranial treatment, has the option for day/nighttime settings for stimulation, and allows additional treatment using a magnet. DBS can be a good option for people who live far from a provider who manages their device as it provides the option of remote programming and gives individuals more control over marking events or multiple event types. BrainSense software allows for more individualized treatment settings and is paving the way for increased adaptive DBS programming options. RNS can be a good option for individuals who can update their data regularly and commit to in-person appointments. The ECoG data collected allow for individualized programming of seizure detectors and stimulation settings to target seizure onset.
Dual device therapy with VNS and DBS or RNS has been reported to be safe and effective, possibly with a synergistic effect.26 People who have not responded adequately to VNS alone may respond to dual device therapy.26,27 Important future directions include identifying preoperative biomarkers to guide optimal device type and placement for effective seizure control in LGS,28 conducting larger prospective trials of neuromodulation devices in people with LGS, and evaluating the potential benefits of new programming, forecasting, and adaptive technologies.
Conclusion
Neuromodulation is increasingly being used as a treatment for LGS, with numerous studies now reporting on the safety and effectiveness of intracranial (ie, DBS, RNS) and extracranial (ie, VNS) neuromodulation methods. Neuromodulation should be considered for all individuals with LGS who do not achieve sufficient seizure control with pharmacologic treatment or dietary therapy or who are not suitable candidates for resective surgery. Although the optimal stimulation target remains under investigation, the centromedian nucleus has demonstrated substantial efficacy, with responder rates consistently >50% in VNS and 60% in both DBS and RNS, with some patients even becoming seizure free.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Epilepsy & Seizures
Pitfalls in Management of Intracranial Hypotension
Rohit Kushwah, MD; Sarbesh Tiwari, DMRohit Kushwah, MD; Sarbesh Tiwari, DM - Epilepsy & Seizures
Treatment of Developmental/Epileptic Encephalopathy With Spike-Wave Activation in Sleep
Lekha M. Rao, MD; Robert C. Stowe, MDLekha M. Rao, MD; Robert C. Stowe, MD - Epilepsy & Seizures
Pharmacologic Management of Behavioral Challenges and Psychiatric Comorbidities in People With Epilepsy
Lauren Waldron, MDLauren Waldron, MD



