Progressive Drug-Resistant Epilepsy & Behavior Changes
Differential Diagnosis
Over the course of AJ’s care, several diagnoses were considered, including focal cortical dysplasia (FCD), perinatal stroke sequelae, hemimegalencephaly, and Rasmussen encephalitis.

FCD
FCDs present as drug-resistant epilepsy and are caused by focal developmental malformations that disrupt the normal cerebral architecture. There are multiple histologic subtypes of focal cortical dysplasia defined by the International League Against Epilepsy task force.1 Typically, individuals with FCD present in the early years of life and often have a positive family history of epilepsy (around 18%) and history of status epilepticus (around 11%). Considering that epilepsy onset is typically in childhood, these individuals can have comorbid cognitive or neuropsychologic challenges. Brain MRI sensitivity is 63% to 98% and can be suggestive of disease, and histopathology confirms the diagnosis.2 The most common MRI findings include focal thickening of the cortex, blurring of the grey–white matter junctions, and abnormal signal intensity in the cortex and subcortical white matter.2 People with FCD have seizures that are commonly refractory to ASMs because of the intrinsic epileptogenicity of the tissue,2 and are therefore good surgical candidates. More than 65% of people with FCD become seizure free after resection, depending on the histopathologic subtype and area of dysplasia.3
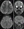
AJ’s MRI was notable for focal sulcal widening suggestive of volume loss, blurred gray-white differentiation, and hazy T2 hyperintensity in the subcortical white matter, which can often be seen in FCD. AJ’s MRI, however, also showed a component of volume loss that was not consistent with FCD. Additionally, the rapid progression of epilepsy with behavioral involvement, including development of multiple seizure semiologies and multifocal seizure onset on EEG, is uncommon in FCD. AJ also did not have histopathologic evidence of FCD.
Perinatal Stroke
Perinatal stroke encompasses a heterogeneous group of cerebrovascular injuries that may be arterial or venous and ischemic or hemorrhagic in nature. Perinatal stroke is defined as occurring between week 20 of gestation and postnatal day 28. Incidence is between 1 in 1,600 to 3,000 live births. Meconium staining is among the most consistently associated risk factors, and incidence is higher in babies identified as male at birth. The etiology of perinatal strokes is broad and incompletely understood. Some evidence points toward thromboembolic events via the placental vasculature leading to an arterial ischemic stroke. Other less common etiologies include congenital cardiac disease and hematologic disorders. Babies may present postnatally with acute symptomatic seizures caused by cerebral injury. Development of epilepsy is typically not immediate and can occur later in life. Sometimes babies can emerge with encephalopathy, irritability, hypotonia or feeding difficulties. Depending on where the injury occurred, affected babies can present with motor weakness or increased tone in the first years of life.4
MRI is the standard for diagnosis of perinatal stroke. In the period after the infarct, there can be loss of cortical tissue in the areas of infarction on T1-weighted imaging. After 1 month, tissue atrophy or cysts can be seen. Diffusion restriction imaging can sometimes show the lesion, although this is dependent on the timing of the injury.5

AJ had risk factors for perinatal stroke during birth, but his imaging findings and clinical progression were not consistent with this diagnosis. Although AJ’s brain imaging showed unilateral volume loss and gliosis as well as signal change on T2-weighted imaging and FLAIR, which can all be consistent with perinatal stroke, he had other imaging changes not consistent with the diagnosis. Additionally, AJ did not have acute symptomatic seizures, and the epilepsy progression and developmental regression that occurred are not consistent with perinatal stroke.
Hemimegalencephaly
Hemimegalencephaly is a rare disorder of unilateral cerebral hemispheric overgrowth that can occur through either a part or the entire cerebral hemisphere.6,7 Hemimegalencephaly may be an isolated occurrence associated with a genetic syndrome, such as tuberous sclerosis complex, or Klippel-Trenaunay syndrome.6 Typically, this diagnosis is characterized as a triad of global developmental delay, epilepsy, and contralateral motor deficits.6 Macrocephaly is typically present in the perinatal period with intractable epilepsy, with onset in the first year of life, that is drug resistant.8 Diagnosis is made with MRI and histopathology. Between 54% and 78% of individuals who have hemispherectomy become seizure free.6
AJ’s progressive epilepsy presentation, imaging, and histopathology were not consistent with this diagnosis. His genetic testing also did not reveal associated genes, although hemimegalencephaly is typically considered to result from somatic mosaicism so that genetic testing from blood or saliva is often nondiagnostic.
Rasmussen Encephalitis
Rasmussen encephalitis is a rare, progressive, immune-mediated disease that is typically characterized by drug-resistant focal epilepsy, cognitive decline, and progressive hemiplegia resulting from hemispheric dysfunction.9,10 Annual incidence of Rasmussen encephalitis is estimated as 2.4 cases per 10 million people age 18 years and younger,9,11 with no clear sexual or ethnic predominance.11 Prevalence studies show there are 2 variants of Rasmussen encephalitis; with childhood onset or later onset. Childhood-onset Rasmussen encephalitis progresses more rapidly to intractable seizures than late-onset.12 Mean age at diagnosis was 4 years and 4 months and 16 years, respectively.12 Approximately 10% of cases have onset in adolescence and early adulthood.10
Formal diagnostic criteria for Rasmussen encephalitis were developed in 2005 by a European Consensus statement (Table) to aid earlier recognition of the disease process and to initiate treatment earlier.13

The natural history of Rasmussen encephalitis includes prodromal, acute, and residual phases. The prodromal phase includes focal epilepsy without associated hemiparesis and minimal clinical deterioration. Typically, about 7 months later, the acute phase includes neurologic deterioration, increased seizure frequency that is difficult to treat, and development of brain atrophy.9,11,13,14 During the acute phase, aphasia (depending on the affected hemisphere), hemiparesis, hemianopsia, and cognitive changes begin.13 In the residual phase, symptoms become stable and drug-resistance of seizures remains.9 Individuals with Rasmussen encephalitis develop multiple seizure semiologies with disease progresses because of ongoing hemicerebral inflammation. EPC occurs in up to 58% of cases.9,12
EEG most commonly shows unilateral hemispheric polymorphic delta waves, intermittent focal slowing, and multifocal ictal and interictal discharges.13-15 Clinical and subclinical seizures occur.11 Although it is atypical, there have been reports of ESES in Rasmussen encephalitis.16 Imaging is typically notable for unilateral focal atrophy, most commonly involving the insular cortex, associated with ipsilateral ventricular enlargement.13,15 There is also associated cortical and subcortical white matter T2 and FLAIR signal changes, usually surrounding the areas of cortical atrophy.15 Histopathology should provide evidence of a chronic inflammation including T-cell dominated encephalitis with activated microglia, and parenchymal macrophages.13,14
Pathogenesis is thought to be immune mediated based on these histologic findings;10 however, no specific pathogen has been associated with this immune activation,10 although antiGluR3 and other autoantibodies have been identified but not found causative.10,13 These autoantibodies have also been seen in noninflammatory focal epilepsies and other nonspecific encephalitides.12 Immunomodulatory treatments have proven unsuccessful for treatment of Rasmussen encephalitis.12
Treatment includes ASMs although most often, Rasmussen encephalitis is drug-resistant. Trials of steroids, immunoglobulin, plasmapheresis, tacrolimus, azathioprine, rituximab and other immunologics have been tried with isolated success, but do not stop seizures or disease progression.10 The highest rates of seizure freedom are seen after hemispheric surgery with as high as a 71% seizure-freedom rate 5 years after surgery.17

AJ met the proposed criteria with clinical assessment, EEG, and MRI findings. He did not develop several of the classic symptoms, however, including progressive cerebral atrophy, EPC, or hemiparesis and quickly transitioned from a prodromal to acute phase. Although AJ initially had a tonic-clonic seizure, within a couple of months he was having multiple seizure types as he progressed to the acute phase. His seizures were resistant to 3 adequately trialed ASMs without success. AJ’s EEG findings were consistent with a focal process, exhibiting focal slowing in the left temporal region and evidence of a focal seizure, although he also developed focal ESES, which is not commonly seen with Rasmussen encephalitis. His stereoEEG was notable for multifocal seizures and discharges. Throughout his 6-month workup he had 3 brain MRI’s that did not show any interval change but consistently showed signal abnormalities in the left anterior insula, left caudate, and frontal lobe with focal asymmetries concerning for focal atrophy.
Considering his atypical presentation, without clear early signs of atrophy or hemiparesis, it was important to have early clinical suspicion and pursue a surgical evaluation. AJ was diagnosed fairly quickly, with time to hemispherectomy of only 6 months. This short interval between initial presentation and hemispherotomy likely accounts for the lack of cerebral atrophy and unilateral hemiparesis, as both of these features take time to develop. AJ’s seizures are now very well controlled with zonisamide, and he has remained seizure free since surgery over approximately 3 months of follow-up.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Epilepsy & Seizures
Promising Results for Investigational Antisense Oligonucleotide Treatment for Dravet Syndrome
Joseph Sullivan, MDJoseph Sullivan, MD - Epilepsy & Seizures
Vagus Nerve Stimulation: Effects Beyond the Nervous System
Stavros Zanos, MD, PhDStavros Zanos, MD, PhD - Epilepsy & Seizures
Management of Poststroke Epilepsy: An Update
Yuki Kawamura, MS; Nitya Beriwal, MBBSYuki Kawamura, MS; Nitya Beriwal, MBBS