Health Care Disparities in Alzheimer Disease Diagnosis and the Use of Amyloid-Targeting Treatments
Disparities in Alzheimer disease care persist, and people from underrepresented racial, ethnic, or socioeconomic groups face considerable barriers to early diagnosis and access to disease-modifying anti-amyloid therapies.
Alzheimer disease (AD), which affects an estimated 6.7 to 6.9 million older adults in the United States,1 is not a distant threat. Without significant intervention, this number is projected to explode to nearly 13.8 million by 2060.1 The field of cognitive neurology continues to make exponential advances, including diagnostic AD biomarker tests and disease-modifying therapies (DMTs) targeting amyloid, such as lecanemab (Leqembi; Eisai, Nutley, NJ) and donanemab (Kisunla; Eli Lilly, Indianapolis, IN). For individuals to access these novel treatments, clinicians must accurately identify cognitive impairment caused by AD early in the disease course.
Equally important is recognizing and addressing the disparities that affect people from underrepresented racial, ethnic, or socioeconomic groups in this field. Among older adults, ~19% of Black individuals and 14% of Hispanic individuals have AD, compared with 10% of White adults. Considering the higher rate of undiagnosed cases among Black and Hispanic populations, these percentages may be higher. In addition, there is sparse data on the prevalence, incidence, and specific risk factors of the disease in people of Middle Eastern, North African, or Native American origin, or among people from more than 1 racial or ethnic group. The cause of low diversity in AD research is multifactorial and includes distrust in medical research due to historical injustice, lack of awareness and outreach, financial and logistical barriers for travel or dedicated time to participate, strict exclusion criteria that may disproportionally exclude non-White participants, limited access to academic research centers, and cultural and language barriers.2
Geography also affects the way people seek and receive care. Although a large percentage of people from underserved populations live in urban areas which is where many neurologists practice, individuals still encounter logistical obstacles accessing these specialists, including socioeconomic stressors (eg, financial barriers, underinsurance, employment insecurity).1,2 Rural America has become more heterogeneous, with the percentage of non-White residents reaching 24% of the rural population.3 This population faces added barriers to accessing AD and dementia care, including limited availability of specialty and academic medical centers (Figure 1).
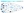
Figure 1. Sequential barriers that contribute to disparities in receiving Alzheimer disease anti-amyloid therapies.
Barriers to Early Detection
Early detection of cognitive impairment is paramount to receive maximum benefit from DMTs, but there are disparities in access to timely and accurate diagnosis. A systematic review examining disparities in dementia diagnosis found evidence of delayed referrals, higher rates of misdiagnosis, and delay in diagnosis in people from underrepresented racial or ethnic groups compared with non-Hispanic White individuals.4
Neuropsychologic testing, a crucial part of the cognitive assessment, can also lead to diagnostic inaccuracies and disparities for non-White individuals. For example, bilingual individuals may perform worse on verbally-mediated tests depending on their level of linguistic adaptation (ie, the ability to adjust and effectively use a second language in different contexts) regardless of level of education. Cultural differences and lack of formal education for immigrants from underdeveloped countries may also result in misdiagnosis.5 All these factors, along with the general use of brief cognitive tests that lack cultural sensitivity or multilingual validation, may lead to inaccurate diagnosis. The Mini-Mental State Examination and the Montreal Cognitive Assessment are commonly used screening tools at bedside, but their effectiveness varies across people with diverse linguistic and cultural backgrounds. Norms developed in affluent, English-speaking populations are not always applicable across different sociocultural contexts.6-11
To receive amyloid-targeting treatments (ATTs), individuals must meet criteria for mild cognitive impairment or mild dementia due to AD. Meeting these criteria requires biologic confirmation of AD,12,13 determined by severity of cognitive impairment. Biomarker testing includes amyloid and tau positron emission tomography (PET), cerebrospinal fluid (CSF) amyloid and tau, and blood-based biomarkers (eg, p-tau217).14 The shortage of trained providers may restrict access to CSF collection, and limited resources and logistic challenges in handling and transporting samples to offsite locations can further hinder availability, especially in rural areas. Barriers associated with amyloid PET tests include availability, interpretation, and cost. Studies have found lower amyloid deposition in Black individuals, Hispanic individuals, Asian individuals, and Pacific Islanders compared with non-Hispanic White individuals, which may render false-negative results and delay diagnosis and care.15 Misdiagnosis may also result from geographic limitations (ie, lack of access to specialized centers that offer amyloid PET tests) and the absence of neuroradiologists trained in a systematic approach to interpreting the amyloid PET images.
Blood-based p-tau217 is a useful biomarker for detecting AD pathology. It effectively predicts brain amyloid levels beyond the range of CSF amyloid tests, and blood tests are more easily obtained and less costly than lumbar punctures or PET scans. A limitation is that its accuracy has been tested mostly in non-Hispanic White individuals (>89%).16 One p-tau217 validation study population included >96% White participants for each category, and <2% Black, Asian, or Hispanic or Latino participants.17 This disparity in research representation for people from underrepresented racial or ethnic groups can lead to a poor understanding of the true disease pathophysiology in these individuals and a lack of confidence around plasma biomarker interpretation. In addition, conditions that affect plasma biomarker interpretation (eg, chronic kidney disease, obesity) are more prevalent in non-White populations, which may further limit plasma AD biomarker clinical utility in these individuals.18
Hurdles in Access to Treatment
Even if an AD diagnosis is made promptly, studies report less use of medications that have been on the market for close to 3 decades (eg, cholinesterase inhibitors) in people from underrepresented racial or ethnic groups compared with non-Hispanic White individuals.4 There will likely be a similar trend for ATTs. There was limited representation of non-White populations in the clinical trials for lecanemab and donanemab (Figure 2). Both clinical trials showed a reduction of amyloid burden and slowing of cognitive and functional decline, but it is unclear how generalizable these results are to people from racial or ethnic groups that were underrepresented in the trials. Transparent communication about these facts is important to foster trust.

Figure 2. Racial and ethnic representation in the clinical trials for donanemab and lecanemab compared with the US population.
Financial Constraints
AD can pose a high economic burden from the diagnosis to the treatment phase. In some circumstances an amyloid PET scan is crucial for biomarker confirmation of an AD diagnosis. Although Medicare provides coverage, individuals can end up paying as high as 20% in copays. Plasma biomarkers are less costly to obtain than PET scans but can still cost hundreds of dollars, as there is no specific reimbursement for these tests.
The Centers for Medicare & Medicaid Services covers ATTs for eligible individuals,19 but coverage through private insurers is inconsistent. Some insurers require prior authorization and others classify these treatments as investigational and refuse coverage altogether. The unpredictability of approvals means individuals may initially be covered only to later face denials, leaving them with unexpected financial burdens. For uninsured, underinsured, or noncovered individuals, out-of-pocket costs can exceed $1500 per infusion, including administration fees. Even for Medicare beneficiaries, the standard 20% coinsurance can reach ~$300 per infusion if deductibles have not been met. Clinicians should also consider the logistical demands of this treatment, including access to intravenous infusion resources, which may be limited in community hospitals; the need for ongoing imaging to monitor for side effects; and travel time to treatment centers, which disproportionately affects patients and caregivers in rural areas.20 Over time, these expenses create considerable financial strain, disproportionately affecting people from underrepresented groups who already experience health care inequities.
Suggestions for a More Equitable Future
The logistics of and high costs associated with ATTs exacerbate existing disparities in AD care. Efforts must be made to mitigate these disparities. Expanding research to better understand biologic variability among diverse populations is paramount. There is evidence that a community-engaged approach can improve the integration of people from underserved populations into clinical trials.21 Possible practical strategies include outreach to trusted community leaders who are knowledgeable about the sociocultural values associated with different racial and ethnic groups, and the selection of community advisors that can partake in the creation of recruitment materials and spread of information.
Funding increases are needed to support research specifically addressing disparities and understanding biologic diversity in AD, such as opportunities offered by the Alzheimer’s Association.22 Collaboration between academic centers and community hospitals can facilitate data collection from individuals who may not typically access academic institutions. In addition, smaller hospitals can benefit from having remote access to resources of larger institutions, such as neuropsychologists, neuroradiology services, and administrative support. The Alzheimer’s Network (ALZ-NET)23 is an initiative in the United States that provides free enrollment for health care providers to access educational resources such as standardized protocols and guidelines for the diagnosis and treatment of AD. Coverage with Evidence Development is an initiative through which Medicare beneficiaries can access real-world research opportunities while receiving treatment. This is a valuable approach to increase representation of people from underrepresented racial, ethnic, or socioeconomic groups in postmarketing research, as it promotes access to these treatments.24
Strategies to improve access to care include expanding and strengthening the dementia-capable health care workforce due to the shortage of trained specialists. One strategy is the use of care navigators (ie, individuals trained to address barriers to dementia care and who can assist individuals and families with navigating the logistics of referrals to neurologists and subsequent care). This approach is particularly valuable in supporting people from underserved populations and improving equitable access to care. Care navigators may help coordinate transportation and appointments, provide cultural and language support, and help navigate insurance logistics.25 Outreach to community resources, such as local Area Agencies on Aging, can also help individuals receive supports that will improve their access to dementia care. Leveraging technology (eg, telemedicine appointments for cognitive screenings), using available translations of the Mini-Mental State Examination or Montreal Cognitive Assessment, and increasing the number of multilingual providers can help expedite diagnosis and referral.
Economic challenges which limit access to ATTs must be addressed. Partnering with organizations that serve diverse communities and can establish financial support for ATTs is a viable solution. Some anti-amyloid drug manufacturers offer financial support for individuals who qualify. Care navigators or social workers are imperative for helping individuals navigate these issues and obtain financial assistance.
Conclusion
Disparities in the diagnosis and treatment of AD disproportionately affect people from underrepresented racial, ethnic, or socioeconomic groups due to structural, socioeconomic, and systemic barriers. The accessibility of novel diagnostic tools and ATTs remains limited for many individuals due to underrepresentation, geographic or financial constraints, or health care infrastructure deficiencies. Addressing these disparities requires a multifaceted approach, including increased racial, ethnic, and cultural representation in clinical trials, improved access to culturally and linguistically appropriate diagnostic tools, expansion of community-based research efforts, and policy changes to ensure equitable coverage and affordability of treatment. Without these targeted interventions, existing health care inequities will persist, limiting the benefits of advancements in AD care. Clinicians must be aware that these disparities exist and should work with a multidisciplinary team and community resources to ensure that any social determinants of health limiting their patients’ access to dementia care are addressed.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Movement Disorders
Diagnosis and Management of Dementia and Psychosis in Individuals With Parkinson Disease
Jorge Patino, MDJorge Patino, MD - Movement Disorders
Diagnosis and Management of Dementia and Psychosis in Individuals With Parkinson Disease
Jorge Patino, MD; Abhimanyu Mahajan, MD, MHS, FAANJorge Patino, MD; Abhimanyu Mahajan, MD, MHS, FAAN - Neurology in Motion
Factors Influencing the Selection of Amyloid PET Tests for Alzheimer Disease
Suzanne E. Schindler, MD, PhDSuzanne E. Schindler, MD, PhD



