A Practical Approach to Triaging Symptomatic Amyloid-Related Imaging Abnormalities
The authors present a framework for recognizing, triaging, and managing symptomatic amyloid-related imaging abnormalities in people receiving amyloid-targeting treatments.
The approval of amyloid-targeting treatments (ATTs) and their subsequent clinical use have reshaped the management of symptomatic early-stage Alzheimer disease (AD). Unlike previously available symptomatic treatments, these disease-modifying monoclonal antibodies remove amyloid-β from the brain in an attempt to slow cognitive and functional decline.1 However, their use has introduced new clinical challenges, including the management of amyloid-related imaging abnormalities (ARIAs) on brain MRI scans, which are frequently observed in treated individuals.2
Neurologists and other providers who treat people with ATTs need to incorporate best practices, including what is known from research trials, to manage ARIAs. This article aims to provide clinicians with a practical guide for identifying and managing ARIAs and distinguishing them from infusion-related reactions (IRRs), and an overview of the broader complications associated with ATTs.
Classification and Clinical Presentation of ARIAs
ARIAs represent a spectrum of MRI-detectable changes observed in people treated with ATTs, classified into 2 main types: ARIAs with edema or effusion (ARIA-E) and ARIAs with hemosiderin deposition (ARIA-H).
ARIA-E (Figure 1) is characterized by vasogenic edema or sulcal effusion, best seen on fluid-attenuated inversion recovery (FLAIR) MRI sequences. ARIA-E may be seen on MRI scans with no associated clinical symptoms. If symptoms occur, they vary in nature and typically are seen when the amount of edema on the MRI scans involves >5 cm of brain tissue. Common complaints associated with ARIA-E include headaches, confusion, nausea, vomiting, dizziness, visual disturbance, tremor, balance, or gait disturbance. In some cases, ARIA-E can cause seizures or focal neurologic deficits, such as weakness, sensory loss, or other cognitive or language problems.
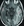
Figure 1. Axial fluid-attenuated inversion recovery MRI scan demonstrating extensive bilateral temporal-parieto-occipital vasogenic edema (red arrows), consistent with amyloid-related imaging abnormalities with edema, in an individual receiving anti-amyloid therapy. The individual presented with confusion and headache. Clinical and radiologic resolution occurred over several weeks.
ARIA-H (Figure 2) is characterized by cerebral microhemorrhages (<1 cm) and superficial siderosis (accumulation of hemosiderin on the surface of the brain [Figure 3]) and is best visualized on T2-weighted or susceptibility-weighted MRI sequences. These microhemorrhages can co-occur with ARIA-E, but also may be seen in isolation. ARIA-H is typically not associated with clinical symptoms, but in rare cases (.5%–.7%) may manifest as a macrohemorrhage (>1 cm) and is often symptomatic. Microhemorrhages can be difficult to discern from normal blood vessels passing through the MRI slice. Some radiologists describe these MRI results using terms such as “definite microhemorrhage” or, if uncertain, a “possible microhemorrhage.” These can be noted and followed up on subsequent MRI scans. Cerebral microhemorrhages and superficial siderosis can also be seen in people with AD who have not received ATT, likely reflecting underlying cerebral amyloid angiopathy.2,3

Figure 2. Susceptibility-weighted MRI demonstrating severe amyloid-related imaging abnormalities with hemosiderin deposits in an individual treated with anti-amyloid therapy. Numerous hypointense foci, consistent with cerebral microhemorrhages, are seen in bilateral cortical and subcortical regions (red arrows). This individual also exhibited concurrent amyloid-related imaging abnormalities with vasogenic edema on fluid-attenuated inversion recovery imaging and presented clinically with headache and confusion, illustrating a symptomatic case of amyloid-related imaging abnormalities with hemosiderin as well as edema.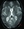
Figure 3. Axial MRI showing superficial siderosis (red arrow). This individual on anti-amyloid therapy had no clinical symptoms.
ARIAs typically occur within the first 6 months of treatment initiation. ARIA-E resolves within 12 to 16 weeks on MRI FLAIR images; ARIA-H remains visible in subsequent imaging.4
Recent data from donanemab (Kisunla; Eli Lilly, Indianapolis, IN) clinical trials (TRAILBLAZER-ALZ [NCT03367403] and TRAILBLAZER-ALZ2 [NCT04437511]) highlight that ARIA frequency varies by therapy and patient profile. For donanemab, ARIA-E occurred in 24.4% of treated participants, with 5.8% being symptomatic, and ARIA-H occurred in 31.3%. APOE ε4 allele carriers, particularly those with homozygosity, faced the highest risk (41.7% ARIA-E incidence).5
The phase 3 Clarity AD clinical trial (NCT03887455) data for lecanemab (Leqembi; Eisai, Nutley, NJ) revealed that ARIA-E occurred in 12.6% of treated participants (symptomatic in 2.8%) and ARIA-H occurred in 17.3%. Again, ARIA-E incidence was highest in APOE ε4 allele carriers, particularly those with homozygosity (45%), compared with those with heterozygosity (19%) or noncarriers (13%).6 Baseline factors, such as microhemorrhages, superficial siderosis, and elevated amyloid burden, further increased susceptibility for ARIAs, underscoring the need for individualized risk assessment before initiating ATTs. This assessment should include detailed review of baseline MRI with FLAIR and susceptibility-weighted imaging sequences, APOE genotyping to identify APOE ε4 carriers, and whether individuals are concomitantly prescribed anticoagulation therapy.5
There are no head-to-head comparison studies between donanemab and lecanemab, and any comparison of ARIA rates noted in clinical trials associated with their Food and Drug Administration approval should be viewed with caution. The populations used in the phase 3 clinical trials for donanemab and lecanemab differed regarding stage of disease, with more participants having mild cognitive impairment due to AD in the lecanemab trials and more with mild AD dementia in the donanemab trials.6,7 It is likely that many factors play a role in the risk of ARIAs.2 A phase 3b study demonstrated that a gradual titration strategy of donanemab led to a 41% relative risk reduction in ARIA-E at 24 weeks compared with the standard dosing, with a greater reduction observed in APOE ε4 allele carriers with homozygosity (from 57% to 19%). This suggests that dosing protocols themselves may also influence ARIA risk independently of patient-related factors.8 Moreover, recent findings have shown that elevated mean blood pressure is independently associated with a higher risk of ARIA-E in people treated with donanemab, highlighting the importance of blood pressure control in mitigating risk.9
Symptomatic ARIAs vs Infusion Reactions
Managing individuals on ATTs requires distinguishing among acute hypersensitivity reactions, IRRs, and delayed ARIAs, which differ in pathophysiology and clinical implications (Table). Hypersensitivity reactions—such as angioedema, bronchospasm, and anaphylaxis—require emergency treatment. IRRs are immediate hypersensitivity responses occurring during or shortly after infusion (up to a few days later) and may present with fever, chills, aches, joint pain, flushing, nausea, vomiting, blood pressure changes, and oxygen desaturation.4 These reactions occurred in 24.5% of participants receiving lecanemab (mostly with the initial infusion) but only in ~10% of participants receiving donanemab. IRRs are usually mild to moderate in severity and can be managed with infusion rate adjustments and preinfusion medication (eg, antihistamines, acetaminophen, NSAIDs, corticosteroids).10

In contrast, ARIAs involve delayed vascular and inflammatory changes due to amyloid clearance, emerging weeks to months after treatment begins. Most ARIA cases are asymptomatic and detected on the recommended safety MRI scans. Symptomatic ARIA-E typically occurs much later than IRRs—71% within 3 months of lecanemab and 53.8% within 3 months of donanemab.5,6 Regarding management, IRRs require acute symptom control, whereas ARIAs require radiographic monitoring, potential dose interruption, and treatment with steroids for moderate to severe cases. These differences highlight the need for structured monitoring and individualized risk assessment.2,6,11
Management Strategy for Symptomatic ARIAs
Step 1: Identification and Timing
Clinicians should maintain high suspicion for ARIAs in people presenting with new neurologic or other clinical symptoms within 6 months of an ATT infusion.
Step 2: Immediate MRI Assessment
An MRI scan with FLAIR and susceptibility-weighted sequences should be obtained. Imaging helps confirm or exclude ARIA-E and ARIA-H and helps rule out other diagnoses, such as strokes, infections, or tumors.
Step 3: ARIA Severity Grading
Clinical trials provide a useful severity grading framework for ARIAs.
For ARIA-E:
- Mild: Focal edema (single focus, <5 cm), asymptomatic or mild symptoms
- Moderate: Multifocal edema (≥2 distinct foci of vasogenic edema, each <5 cm but in multiple lobes or hemispheres) or symptoms interfering with daily activities
- Severe: Extensive edema, mass effect, or severe clinical symptoms (>5 cm of contiguous brain tissue, may cross multiple lobes or hemispheres, often with sulcal effusion or mass effect)
For ARIA-H:
- Mild: 1 to 4 new cerebral microhemorrhages or 1 area of superficial siderosis
- Moderate: 5 to 9 new cerebral microhemorrhages or 2 areas of superficial siderosis
- Severe: ≥10 new cerebral microhemorrhages, >2 areas of superficial siderosis, or any macrohemorrhage (>1 cm)12
Step 4: Treatment Decisions
- Mild ARIA-E or ARIA-H: Continue treatment with close monitoring or temporarily pause therapy to wait until clinical symptoms, if any, resolve.
- Moderate to severe ARIA-E: Hold therapy until ARIA-E and any clinical symptoms resolve. If symptoms are substantial, initiate corticosteroids (eg, 1 g prednisone for 3 to 5 days followed by a several-week taper).13
- Moderate to severe ARIA-H: Hold therapy until MRI demonstrates radiographic stabilization of ARIA-H (ie, no increase in size or number of ARIA-H findings).
- Severe ARIA-E or ARIA-H: Consider permanent discontinuation of therapy.
Treatment should be discontinued permanently if ≥3 episodes of ARIA occur, regardless of clinical or radiologic severity, and if ARIAs are accompanied by seizures.13
Broader Treatment Considerations in AD
In addition to ARIAs, other factors related to ATTs can lead to complications or barriers that affect patient management.
MRI Monitoring Burden
MRI brain scans at baseline and multiple additional MRI scans to evaluate for asymptomatic ARIAs are required. If there are missed infusions or pauses in therapy due to ARIAs or other reasons, previously scheduled MRI scans need to be adjusted accordingly. Moreover, if clinical symptoms are reported inbetween scheduled MRI scans that may be due to ARIAs, an additional MRI scan will need to be performed to assess for ARIAs. Acquiring MRI scans is resource-intensive and logistically challenging, particularly in community settings. A radiologist familiar with detecting ARIAs is also needed, and access to one may be limited in certain community settings.14 Ideally, individuals should present to a hospital with neurology and EEG access, and emergency physicians must recognize ARIAs and distinguish them from stroke to avoid inappropriate thrombolytic treatment without proper evaluation.
Risk of Anticoagulation
People receiving anticoagulation should generally be excluded from initiating ATTs due to the increased risk of ARIA-H, including microhemorrhages and potentially fatal intracerebral bleeding. Similarly, initiating anticoagulation in people already receiving ATTs is strongly discouraged unless absolutely necessary, given the additive risk of hemorrhagic complications. If anticoagulation needs to be started, ATTs should be stopped.
Findings on baseline MRI scans suggestive of cerebral amyloid angiopathy may increase the risk of intracerebral hemorrhage if ATTs are used.15
Access and Equity
Treatment availability is skewed towards specialized centers with infusion and imaging capabilities. Insurance limitations and racial, ethnic, and socioeconomic disparities may exacerbate access issues.16
Patient Expectations
There is a risk of overestimating the clinical benefit of amyloid reduction with ATT. Education needs to stress that anti-amyloid medications only slow decline. They do not stop decline or improve symptoms.17
Practical Guidelines for Monitoring People on ATT
- Baseline Evaluation. Begin with a 1.5T or high-resolution 3T MRI, including T2-weighted FLAIR and susceptibility-weighted imaging sequences, to identify any existing microhemorrhages (if >4, therapy is excluded) and assess white matter changes. APOE genotyping is crucial for risk assessment. Baseline cognitive testing should also be performed to ensure the patient is in the early symptomatic stage of AD and to aid in future symptom tracking.1
- Early Imaging Surveillance. ARIAs often emerge early in treatment (in the first 2 to 6 months). For the monthly donanemab infusions, MRIs are recommended prior to the second, third, fourth, and seventh infusions. For the biweekly lecanemab infusions, MRIs are recommended prior to the fifth, seventh, and fourteenth infusions.1
- Active Symptom Monitoring. Educate patients and caregivers on expected timing and types of adverse effects and that most ARIAs are asymptomatic. Prompt symptom reporting is essential. Nearly half of symptomatic individuals present with headaches.18
- Reporting ATT Use. Patients should always report (verbally or through a medical alert card or bracelet) to emergency providers, in addition to other health care providers, that they are using ATTs, especially before starting anticoagulants or thrombolytic agents.14
- Triage and Response Protocols. Institutions should establish clear workflows for managing suspected ARIAs. This includes prompt MRI evaluation, temporary treatment suspension for moderate to severe radiographic ARIAs (as described previously), and neurologist referral for seizures or focal deficits. Most ARIA-E cases (82%–98%) resolve within 4 months without intervention, but corticosteroids may be used in cases of severe edema.11
- Shared Decision-Making and Risk Disclosure. Especially in people with 2 copies of the APOE ε4 allele (APOE ε4/ε4), clinicians should clearly explain the elevated ARIA risk vs potential benefits. Counseling should cover the intensity of monitoring—typically requiring 4 to 6 MRIs in the first year—and the lack of long-term outcome data.1,19
Conclusion
ATTs represent a considerable advance in early symptomatic AD treatment. However, ARIAs pose a unique set of clinical challenges that require timely recognition, accurate triage, and thoughtful management. Differentiating ARIAs from infusion reactions is essential for safe and effective therapy. By adopting protocols and patient-centered approaches, neurologists can integrate these therapies into practice while minimizing risks and maximizing clinical benefits. n
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Movement Disorders
Diagnosis and Management of Dementia and Psychosis in Individuals With Parkinson Disease
Jorge Patino, MDJorge Patino, MD - Neurology in Motion
How to Communicate the Results of Alzheimer Disease Biomarker Tests with Patients
Suzanne E. Schindler, MD, PhDSuzanne E. Schindler, MD, PhD - Neurology in Motion
Factors Influencing the Selection of Amyloid PET Tests for Alzheimer Disease
Suzanne E. Schindler, MD, PhDSuzanne E. Schindler, MD, PhD



