Critical Bilateral Carotid Artery Disease Presenting as Hemichorea
Vascular imaging can be an important test in the diagnostic workup of adult-onset hemichorea, even in the absence of structural brain lesions.
Case Presentation
DJ, age late 70s, presented to the outpatient clinic with a 1-year history of subtle jerking of the right hand that occasionally led to dropping objects. No other symptoms, including transitory focal neurologic deficits, were reported. Medical history was notable for human immunodeficiency virus (HIV) infection for >25 years, with undetectable viral RNA, and CD4 count >1000 cells/mm3; low-grade urothelial bladder carcinoma treated with mitomycin C, which had been in remission for 7 years; right-hand trauma 20 years previously; and chronic obstructive pulmonary disease. DJ had a 100 pack-year smoking history but had quit smoking 8 years previously. There was no previous use of antipsychotics, history of drug abuse, or family history of involuntary movements or neurologic disorders.
On initial observation, DJ presented with repetitive, involuntary, irregular, purposeless, nonrhythmic movements of the right hemibody with brachial predominance, sparing the face (see Video 1). This hemichorea was apparent throughout the interview in the upper limb, exacerbated by distracting maneuvers and to a lesser extent in the lower limb through gait observation using the Fogs test. Muscle strength, sensory evaluation, and deep tendon reflexes were unremarkable; positive clonus, Babinski, or Hoffman signs were not present.
Video 1. Involuntary movements of the right hemibody with brachial predominance observed during initial observation.
Diagnostic Process
Considering the syndromic diagnosis of subacute adult-onset hemichorea and DJ’s history, the following diagnostic hypotheses were considered: a structural central nervous system (CNS) lesion, particularly in contralateral basal ganglia, including the subthalamic nucleus, more likely vascular (eg, stroke), but also possibly tumoral (eg, urothelial carcinoma metastatic disease, HIV-related CNS lymphoma) or inflammatory (eg, CNS HIV escape infection; opportunistic infection; other autoimmune, demyelinating, or paraneoplastic condition; indolent infections, including variant Creutzfeldt-Jakob disease); CNS toxicity (from anticholinergic medication for chronic obstructive pulmonary disease or a possible recreational drug); metabolic disturbance (eg, nonketotic hyperglycemia, acquired hepatocerebral degeneration, vitamin B12 deficiency, hyperthyroidism); or a functional disorder.
Laboratory workup results were unremarkable (Table), and there were no parenchymal changes evident on brain MRI (Figure 1A). A magnetic resonance angiographic study, however, revealed a left internal carotid artery (ICA) occlusion and a right ICA critical stenosis (Figure 1B). Perfusion studies showed global and symmetric hypoperfusion (Figure 1D), and Doppler ultrasound revealed bilateral dampened middle cerebral artery (MCA) velocities.

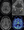
Figure 1. Axial T2-weighted fluid-attenuated inversion recovery image shows no basal ganglia structural lesions (A). Magnetic resonance angiography demonstrates right internal carotid artery critical stenosis (white arrow) and absence of left internal carotid artery blood flow signal (yellow arrow) (B). Axial postgadolinium T1-weighted image shows no enhancing lesions that could suggest brain metastases (C). Cerebral blood flow demonstrates global brain hypoperfusion (D).
Considering the severe hypoperfusion, a decision to treat was made using an endovascular approach. A 90% right ICA stenosis was confirmed at angiography (Figure 2), and angioplasty with stent and balloon was performed, with satisfactory recanalization, and marked hemodynamic improvement.

Figure 2: Digital subtraction angiography showing a severe stenosis of the right ICA (A), and the result after balloon angioplasty and stenting (B). Pre-stent images of the injection on the right common ICA show a marked delay in perfusion of the ICA territory (C). After stent, there was a significant improvement of the cerebral hemodynamics on the right ICA territory, and also, collateral flow to the left ICA territory, through the anterior communicating artery (D).
Case Resolution
Immediately following right ICA angioplasty and stenting (see Video 2), there was improvement seen in chorea with substantial gradual improvement at 3 months (see Video 3) and 6 months (see Video 4). Eventually, only barely noticeable hand movements with distracting maneuvers persisted. Doppler ultrasound showed normalized velocity of the right MCA and improved velocity of the left MCA.
Video 2. Improvement in hemichorea immediately following right ICA angioplasty and stenting.
Video 3. Improvement in hemichorea 3 months after right ICA angioplasty and stenting.
Video 4. Improvement in hemichorea 6 months after right ICA angioplasty and stenting.
Discussion
Hemichorea consists of irregular, purposeless, arrhythmic, nonstereotyped involuntary movements that flow from 1 body part to another.1 Hemichorea is widely accepted as a manifestation of basal ganglia dysfunction explained by the classic model of basal ganglia function in which the decreased inhibitory input of the globus pallidus pars interna to the thalamus results in facilitation of the thalamocortical motor drive.1 There is a wide differential diagnosis for hemichorea,2 and although acute stroke is the most common cause of acquired chorea, chorea itself is a relatively rare stroke symptom.3 Few case reports advocate for ICA stenosis to be considered in chorea workup, suggesting a lesion in the contralateral basal ganglia or related structures as a key factor.4-7 Despite several localizations being reported, network mapping appears to overlap at the posterolateral putamen, and in the absence of infarct, chronically disrupted perfusion should be considered.3 Carotid occlusive disease compromises blood flow in perforating lenticulostriate branches, possibly leading to basal ganglia ischemia or reduced perfusion.7 This mechanism is similar to what is described for limb-shaking transient ischemic attacks; however, the phenomenology described is different. DJ’s movement disorder was not rhythmic, brief, or paroxysmal.8 Moreover, in our case, we could not establish a relationship between symptom onset or aggravation and position (ie, orthostatism).
Reduced perfusion was suspected only because of the angiographic findings, highlighting the need to include an angiogram in the workup for hemichorea, at least in individuals with vascular risk factors, even in the absence of evidence of parenchymal ischemic lesions.
The differential diagnosis in our case was influenced by DJ’s medical background. The history of urothelial carcinoma raised the concern that chorea could be related to CNS structural metastatic disease9 or a paraneoplastic syndrome, although this is rare with this neoplasm and more likely to present as cerebellar degeneration or Lambert-Eaton myasthenic syndrome.10 Structural CNS disease associated with HIV CNS viral escape11 is described even with low-level viremia, and the possibility of opportunistic infection or CNS lymphoma was also on the table, even though there was no apparent immunosuppression. Other autoimmune, demyelinating, and paraneoplastic diseases were set aside after negative serum autoantibody testing, especially in the absence of structural changes on MRI and in the presence of a suitable alternative diagnosis.12 CSF studies were therefore not pursued. Variant Creutzfeldt-Jakob disease was a remote hypothesis to begin with, given the temporal evolution and relatively benign nature of the symptoms.
Every metabolic disturbance with the potential to cause chorea was excluded based on laboratory workup. Nonketotic hyperglycemia and acquired hepatocerebral degeneration were unlikely in the absence of a history of diabetes or liver dysfunction, respectively. Hyperthyroidism can cause hemichorea in the absence of structural abnormalities3; however, there were no accompanying symptoms, and this was excluded by the laboratory workup. Wilson disease was not specifically investigated considering the MRI results and the presence of an alternative diagnosis.
CNS toxicity due to anticholinergic or recreational drug use or a functional neurologic disorder were diagnoses of exclusion in this context.
The entities considered were reasonably excluded considering the case evolution, especially in light of the clinical improvement after stenting.
Some recommended approaches1 suggest testing all individuals with adult-onset chorea for Huntington disease (HD); however, considering the absence of prodromal HD symptoms (motor, cognitive, or behavioral), lack of family history, and presence of hemichorea, which is considered a negative predictor for HD,13 we did not perform genetic testing.
The clinical evolution in this case supports the hypothesis that the pathophysiology of hemichorea associated with carotid artery stenosis is hemodynamic compromise of basal ganglia and highlights the importance of pursuing vascular imaging investigation in people with hemichorea despite normal structural neuroimaging results and in the absence of other clinical signs.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Alzheimer Disease & Dementias
Medical Aid in Dying: An Overview for Neurologists
Christina L. Vaughan, MD, MHS; Nicole Sucre, PsyDChristina L. Vaughan, MD, MHS; Nicole Sucre, PsyD



