Stroke in a Young Adult:
Immunomodulation-Responsive Bilateral Supraclinoid Carotid Artery Vasculopathy Secondary to Systemic Lupus Erythematosus
Immunomodulation-Responsive Bilateral Supraclinoid Carotid Artery Vasculopathy Secondary to Systemic Lupus Erythematosus
Stroke occurs in 3% to 20% of people with systemic lupus erythematosus (SLE) and accounts for 15% of deaths in people with SLE.1 Central nervous system (CNS) vasculitis secondary to SLE is extremely rare and mainly affects the small vessels, according to histopathologic studies. The pathophysiology of CNS vasculitis due to SLE is thought to be secondary to immune complex deposition triggering perivascular inflammation, endothelial proliferation, and hyalinization. This can result in ischemic stroke or microinfarcts causing seizure or cranial nerve palsies.2 A paucity of data exist on CNS vasculitis and vasculopathy in SLE, especially involving cerebral large vessels.3,4 This treatable condition is important to include in the differential diagnosis, especially for strokes in young adults or individuals with SLE or related autoimmune history. We present a rare case of ischemic stroke in the setting of large-vessel vasculopathy in an individual with SLE.
Case Presentation
CP, aged early 20s, who had been diagnosed with SLE 12 years previously, presented as a transfer from an outside hospital for stroke workup. CP had presented to the outside hospital after experiencing right-sided weakness and imbalance leading to a fall, vision changes, and confusion. CP had a transient episode of monocular vision loss with associated headache 2 weeks previously that self-resolved, for which CP did not seek medical care. On arrival upon transfer, CP was afebrile, with a blood pressure of 132/88 mm Hg and heart rate of 82 beats per minute. On examination, CP had mild to moderate receptive aphasia, with right arm weakness greater than leg weakness, and right arm and leg ataxia, with a National Institutes of Health Stroke Scale score of 7. The time window to consider intravenous thrombolysis therapy had passed. On the second morning of hospitalization, CP acutely developed bilateral visual deficits, disorientation, personality change with elevated affect, and cognitive deficits, including difficulty with abstract thinking and performing calculations.
In terms of patient history, CP had regular follow up with a rheumatologist since being diagnosed with SLE. The most recent symptomatic flare had occurred 5 years previously, with characteristic malar rash, arthralgias, and fatigue, and a renal biopsy indicated lupus nephritis. CP was treated with cyclophosphamide and steroids, and was then transitioned to hydroxychloroquine and mycophenolate, which had been continued since that time. The patient had developed Budd-Chiari syndrome 2 years previously, which was treated with warfarin. Hypercoagulability workup had been conducted at that time and results are detailed in the following section. Because of resolution of clot burden upon reassessment, anticoagulation had been discontinued after 1 year (ie, 1 year before the current presentation).
Diagnostic Process
MRI performed at the outside hospital before transfer demonstrated bilateral middle cerebral artery (MCA)/anterior cerebral artery (ACA) and MCA/posterior cerebral artery watershed infarcts, with greater involvement on the left side, corresponding to the clinical presentation (Figure 1). No stenosis, occlusion, or other remarkable findings were visualized on initial CT angiography. Acute vision loss and worsening cognition on the second day of hospitalization prompted repeat MRI, which showed extension of parieto-occipital infarcts, and angiogram was performed. Cerebral angiogram revealed bilateral supraclinoid internal carotid artery (ICA) stenosis (right, 79%; left, 61%), without distal medium or small-vessel irregularity or beading to suggest a diffuse vasculitic process (Figure 2).

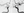
Transcranial Doppler ultrasound demonstrated severe to critical stenosis vs spasm in the right MCA based on velocity 172 cm/s (normal range, 40 to 80) and MCA to ICA ratio of 8.6 (normal Lindegaard ratio, < 3), as well as harmonic bruits, which indicate turbulent flow (Figure 3, left). The left MCA had moderate stenosis vs spasm with velocity 158 cm/s and ratio of 4.8, with borderline elevation in the left ACA of 61 cm/s (normal range, 35 to 60). High-intensity transient signals (HITS) were seen in the right MCA window, suggesting microembolization, thought likely to be attributable to artery-to-artery embolism from the stenotic vessels (Figure 3, right).

Lumbar puncture demonstrated 15 white blood cells/μL, a protein level of 41 mg/dL, and a glucose level of 45 mg/dL in the cerebrospinal fluid. Serum studies demonstrated erythrocyte sedimentation rate of 85 mm/hr (elevated), normal C-reactive protein level, hemoglobin A1C 5.7%, low-density lipoprotein level of 86 mg/dL, and thyroid-stimulating hormone level of 2.7 mIU/L. Infectious workup and urine drug screen were negative.
Transthoracic echocardiogram showed normal left ventricular function with ejection fraction of 60% to 65%, no wall motion abnormalities, and late right-to-left shunt, favored to be a pulmonary shunt over patent foramen ovale. Transesophageal echocardiogram was negative for Libman-Sacks endocarditis. Deep venous thromboses were not detected on Doppler ultrasound of the extremities.
Hypercoagulability workup conducted 2 years previously, upon presentation with Budd-Chiari syndrome, had been remarkable for positive DNA double-stranded antibody with elevated titers (1:80) and borderline low protein C level of 60%. Testing had been negative for antiphospholipid, cardiolipin, and beta-2 glycoprotein antibodies; lupus inhibitor; prothrombin 20210; factor V Leiden; increased factor VIII; protein S deficiency; antithrombin deficiency; C3 and C4 levels; dysfibrinogenemia; and JAK2 and methylenetetrahydrofolate reductase gene mutations.
Case Resolution
Although involvement of cerebral large vessels is atypical in lupus vasculitis, CP was treated empirically with pulse dosing of intravenous methylprednisolone followed by oral prednisone taper. Rheumatology was consulted, with initial low suspicion for lupus vasculitis given the rarity of large-vessel involvement and the lack of characteristic cerebrospinal fluid findings of elevated protein or lymphocytic pleocytosis.5 It was imperative to consider simultaneously and treat empirically other potential causes on the differential diagnosis. At first, antiplatelet therapy was initiated with aspirin and clopidogrel for the bilateral ICA stenosis. CP was switched from antiplatelet therapy to anticoagulation, with a heparin bridge to warfarin due to concern for systemic hypercoagulability because of lupus on the differential diagnosis as well as concern for microemboli detected on ultrasound. This treatment had to be paused briefly later during hospitalization because of hemorrhagic transformation, but was restarted before discharge. Serial transcranial Doppler testing demonstrated resolution of HITS during hospitalization and improvement in arterial velocities over the hospital course, with right MCA 96 cm/s, left MCA 90 cm/s, and left ACA 41 cm/s when measured 4 weeks after initial presentation.
CP was discharged to high-intensity inpatient rehabilitation 5 weeks after initial presentation. Examination upon discharge was notable only for mild weakness in right hemibody compared with left (4/5 strength), and improving left inferior homonymous quadrantanopia.
CP followed up in the stroke outpatient clinic and surveillance imaging was performed. Magnetic resonance angiography performed 11 months after initial presentation demonstrated persistent stenoses concerning for early moyamoya (Figure 4). CT angiography at 2 and 3 years after initial presentation demonstrated substantial improvement and almost complete resolution of bilateral supraclinoid ICA stenosis (Figure 5). Transcranial Doppler testing demonstrated continued improvement, with measurements of left MCA velocity 47 cm/s and right MCA 68 cm/s after 2 years.


CP continues to do well neurologically, with a modified Rankin Scale score of 1. The course has been complicated by interval development of generalized tonic-clonic seizures, which are well-controlled on levetiracetam and zonisamide. Two years after initial presentation, CP was found to have nonocclusive venous sinus thrombosis of the right lateral transverse sinus, which resolved with continued warfarin. Per recommendation of hematology, CP will remain on warfarin indefinitely. Hydroxychloroquine has been continued, with recent discontinuation of mycophenolate because of clinical stability and planned pregnancy. CP continues to follow up regularly in clinic with stroke neurology, epilepsy (initially general neurology), rheumatology, hematology, ophthalmology, and primary care.
Discussion
Given the high morbidity and mortality associated with SLE vasculopathy, providers must be aware of this disease. We suspect that SLE vasculitis contributed to the bilateral supraclinoid ICA vasculopathy in the current case. Diagnosis of CNS vasculitis attributable to SLE can be challenging because the radiographic appearance is similar to moyamoya, intracranial atherosclerosis, and reversible cerebral vasoconstriction syndrome (RCVS). We suggest early initiation of immunomodulatory therapy including high-dose steroids if vasculitis is suspected as contributing to vasculopathy in individuals with SLE who present with an ischemic stroke. If there is a high suspicion for secondary CNS vasculitis and infection has been ruled out, immunosuppressant therapy should be considered while evaluation for alternative etiologies (e.g., moyamoya and the other aforementioned conditions, which can appear similar on imaging, especially early in the disease course) is being conducted.
In this case, the cerebral angiogram (Figure 2) demonstrated stenosis from eccentric-appearing filling defects, which could be explained by mural thrombus, vasculitis, or RCVS.6 Appearance on magnetic resonance angiography at 11 months after initial presentation (Figure 4) showed persistent yet more concentric-appearing stenoses, with moyamoya remaining on the differential diagnosis. If the stenosis was attributable entirely to mural thrombus, we would have expected it to have resolved at 11 months with anticoagulation therapy.7 An important limitation of this case is that magnetic resonance vessel wall imaging was not obtained on initial presentation, which would have helped elucidate the nature of the stenoses and demonstrate whether the vessel narrowing was consistent with vasculitis.6,8 Improvement of distal stenoses was demonstrated in the acute period on transcranial ultrasound after pulse dose steroid therapy followed by prolonged oral taper, and improvement of bilateral supraclinoid ICA stenoses was visualized on CT angiogram at 24 months and 36 months. This timeline of improvement suggests a slower process, such as inflammation attributable to vasculitis underlying the stenoses, rather than an acute or subacute process, such as a mural thrombus.7,9 We would not expect such reversal of stenoses if caused by moyamoya or atherosclerosis, and we would have expected a much shorter course of resolution of stenoses in case of RCVS.9 The improvement and time course therefore makes it likely that CP’s presentation was at least partially explained by large-vessel CNS vasculitis.
CNS vasculitis is a rare cause of ischemic stroke even among individuals with SLE. When CNS vasculitis is the cause of ischemic stroke, it is typically in association with infection and rarely as a primary process. The most common causes of stroke in SLE described in the literature are hypercoagulable state, mediated by antiphospholipid antibodies; cardioembolic cause, such as from Libman-Sacks endocarditis; or atherosclerosis, especially in conjunction with hypertension or acceleration from long-term corticosteroid use.10,11 Because of numerous thromboembolic stroke risk factors and pathogeneses, anticoagulation is the cornerstone of secondary stroke prevention in individuals with SLE who present with embolic phenomena.11 In CP, anticoagulation appeared to have made a difference in the acute course, with demonstrated resolution of HITS on transcranial ultrasound. CP developed transient dural venous sinus thrombosis years later, which may have been related to a hypercoagulable state; thus the decision was made to continue anticoagulation indefinitely.
Stroke typically manifests within the first 5 years of SLE diagnosis, whereas CP presented 12 years into the disease course.11,12 Hypertension is the main risk factor associated with stroke in SLE; renal involvement (typically early in the natural history) may exacerbate the risk and contribute further to stroke, along with the higher risk attributable to the hypercoagulable state.12 In a case-based literature review of cerebral medium-sized arteritis in SLE,3 the findings suggested that arteritis progresses insidiously during the active phase of SLE, which can lead to cerebral infarction years later, regardless of SLE disease activity at the time. We postulate that this time course is similar for large-vessel vasculitis or other vasculopathy, and that there may be reason to consider placing medium- or large-vessel vasculopathy higher on the differential diagnosis when stroke presents more than 5 years after SLE diagnosis.
Because of risk of recurrence, and because both SLE and long-term steroids can accelerate atherosclerosis, we propose continued surveillance of vessels with carotid and transcranial ultrasound in individuals with SLE who have had an ischemic stroke. Surveillance thus far in this case has been reassuring, with no recurrence of large-vessel stenoses seen. The distal vessel velocities have improved, and there has been no evidence of recurrent microembolization. CP has not had another stroke. CP has developed epilepsy, but it is unknown whether this is secondary to encephalomalacia from the strokes due to CNS vasculopathy causing new microinfarcts that become epileptogenic foci, or idiopathic. The seizures are well-controlled on levetiracetam and zonisamide, and CP has been clinically stable otherwise.
Whereas large-vessel CNS vasculopathy in the setting of SLE presents a challenging diagnosis, it is a treatable condition that should be considered on the differential for strokes with signs of large-vessel involvement, especially in young adults. Immunosuppression is essential to treatment if vasculitis is present and may involve a prolonged course of recovery of stenoses, whereas anticoagulation should be considered concurrently to treat the more common embolic etiologies of stroke in SLE, such as hypercoagulability. Ongoing surveillance and multidisciplinary follow-up have revealed clinical and radiographic improvement, and CP has no disability from the stroke.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Alzheimer Disease & Dementias
Capacity Determination and Advance Care Planning
Michael Rubin, MD, MA; Anne Lai Howard, JDMichael Rubin, MD, MA; Anne Lai Howard, JD - Alzheimer Disease & Dementias
Medical Aid in Dying: An Overview for Neurologists
Christina L. Vaughan, MD, MHS; Nicole Sucre, PsyDChristina L. Vaughan, MD, MHS; Nicole Sucre, PsyD - Alzheimer Disease & Dementias
Spirituality and Spiritual Care of People With Chronic Neurologic Illness
Sue Ouellette, PhD, MDivSue Ouellette, PhD, MDiv



