Understanding Chiari Malformation Comorbidities
The most common type of Chiari malformations is type I (CM-I), which is characterized by herniation of cerebellar tonsils more than 5 mm below the foramen magnum. CM-I presenting signs and symptoms are caused by compression of the cerebellum, brainstem, and upper cervical spinal cord, and disruption of cerebrospinal (CSF) fluid flow around and through the foramen magnum. A significant minority of those with CM-I are asymptomatic at diagnosis. Those with symptoms typically have headaches, neck pain, dizziness, and nausea. However, because the incidence of migraine, tension-type, and chronic daily headaches in people with CM-I is similar to the general population, cine flow MRI studies are needed to determine whether headaches in a person with MRI evidence of cerebellar tonsil herniation are a symptom of CM-I. In Part 2 of this special report, the most common CM-I comorbidities and potential exacerbation by trauma are discussed.
Several comorbid conditions are associated with CM-I. The most common and serious comorbid congenital anomalies are syringes, scoliosis, and tethered spinal cord syndrome. Hydrocephalus, pseudotumor cerebri, empty sella, polymicrogyria, pineal cysts, and craniosynostosis are less commonly encountered congenital comorbidities of CM-I.
Syrinx
A syrinx is a slit-like cavity within the spinal cord (Figure 1). The classification of spinal cord syringes has been confusing with conflicting nomenclature. In 1 classification system, the term syringomyelia describes a syrinx within the parenchyma of the spinal cord, and hydromyelia describes a syrinx caused by dilation of the central canal. It has been suggested, however, that the term hydromyelia be discarded, and syringomyelia be classified into 5 groups: hydrocephalic, hindbrain-related, nonhindbrain-related, complex, and idiopathic.1 Another classification system, which is most commonly utilized divides spinal cord syringes into 3 separate groups as follows.2
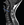
Communicating Syringomyelia
In communicating syringomyelia, previously termed communicating hydromyelia, dilations of the central canal are anatomically continuous with the fourth ventricle and lined completely or partially by ependymal cells. These rarely rupture into the spinal cord parenchyma. In typical communicating syringomyelia, there is generalized enlargement of all 4 cerebral ventricles, and the central canal participates in the hydrocephalic process like a “fifth ventricle.” Causative factors for communicating syringomyelia include postmeningitic and posthemorrhagic hydrocephalus, complex hindbrain malformations (eg, Chiari type II malformation [CM-II] with encephalocele), and Dandy-Walker cysts.
Noncommunicating Syringomyelia
In noncommunicating syringomyelia, previously termed noncommunicating hydromyelia, central canal dilations do not communicate with the fourth ventricle and often have extensive areas of ependymal denuding, paracentral dissection, and intracanalicular septae. Noncommunicating syringomyelia have a propensity for dissection into spinal cord parenchyma and may extend through the pial surface to communicate with the subarachnoid space. Obstructions of CSF pathways at or below the foramen magnum are associated with noncommunicating syringomyelia. Causes include CM-I, basilar invagination, spinal arachnoiditis, extramedullary compressions, tethered cord, and acquired tonsillar herniation.
The spinal canal diameter is 0.05 to 0.1 mm at birth and progressively closes during childhood and adolescence, although it remains patent in 20% to 30% of adults.3 Noncommunicating syringomyelia may thus occur as a result of a normal variant or as a consequence of a CM. As a normal variant, the syrinx is predominantly located centrally in the upper thoracic spine, extending over 3 to 5 segments with a filiform shape.4,5 Criteria for a patent central canal should also include a localized short segment, nonenhancing central medullary cavity, occurring in an unenlarged or only slightly enlarged spinal cord. The cavity should not progress over time and there should be no progressive signs or symptoms specifically related to the spinal cord.6
The prevalence of noncommunicating syringomyelia in CM-I is conflicting and ranges from 12% to 85%.7-11 Age and whether symptoms were present at diagnosis may account for this wide variation. Noncommunicating syringomyelia may be symptomatic or asymptomatic and are often found when a person is evaluated for a possible concussion or cervical spine arthritis. When symptoms are present, the typical clinical presentation is a central cord syndrome characterized by a weakness that affects the arms more than the legs with loss of deep tendon reflexes at the level of the cavity. Persons with symptomatic noncommunicating syringomyelia often exhibit a pattern of “dissociated sensory loss” that involves the preservation of fine touch, vibration, and proprioception with selective loss of pain and temperature sensation. A less frequently encountered sensory pattern is called a “suspended sensory level” in which there is a loss of pain and temperature sensation in the distribution of the lesion with relatively normal pain and temperature sensation above and below the level of the lesion. In the suspended sensory level presentation, vibration and proprioception are spared at all levels.
Progression of noncommunicating syringomyelia symptomatology in CM-I is caused by the action of the cerebellar tonsils, which partially occlude the subarachnoid space at the foramen magnum and act as a piston on the partially enclosed spinal subarachnoid space. This creates enlarged cervical subarachnoid pressure waves that compress the spinal cord from without, not from within, and propagate syrinx fluid caudally with each heartbeat, leading to syrinx progression. Syrinx progression is defined using 1) a change in syrinx anteroposterior (AP) diameter (≥1 mm) or 2) a change in syrinx length (craniocaudal, ≥1 vertebral level).9 Syrinx stability is defined as less than a 1-mm change in syrinx AP diameter and no change in syrinx length. Later in the course of the syrinx, with distortion of various spinal cord pathways, segmental weakness, atrophy, upper motor neuron signs, and autonomic dysfunction may develop.
Primary Parenchymal Cavitations
Previously termed syringomyelia or posttraumatic syrinx, primary parenchymal cavitations are noncommunicating extracanalicular syringes that originate within the spinal cord parenchyma and do not communicate with the central canal or fourth ventricle. Primary parenchymal cavitations are lined with gliotic cells and caused by spinal cord tissue injury (eg, trauma, ischemia/infarction, and spontaneous intramedullary hemorrhage).
Traumatic primary parenchymal cavitations occur in 3% to 4% of spinal cord injuries and in other acute myelopathies. In a person who sustained a back injury and several months later has an MRI finding of a syrinx, the question may arise whether the syrinx represents CM-I–related noncommunicating syringomyelia that dissected into the spinal cord parenchyma because of the trauma or a pre-existing primary parenchymal cavitation. Distinguishing between these 2 possibilities often relies on 2 key observations: 1) whether there were clinical signs and symptoms (eg, weakness, sensory loss, or reflex changes) or imaging evidence (eg, spinal cord edema or bleeding) of a spinal cord injury at the time of the back injury; and 2) how soon after the back injury the syrinx was observed.
The interval between the spinal cord injury and the development of a primary parenchymal cavitation can vary from several months to many years. In a study of 53 people with progressive primary parenchymal cavitations, neurologic deterioration, and/or pain syndromes developed an average of 11 years after the initial trauma (range 0.4-41 years).12 In another study of 449 individuals with primary parenchymal cavitation, the average time between injury and the first symptoms was 7.2 years (range 2 months to 30 years) for complete cord lesions and 7.7 years (range 22 months to 20 years) for incomplete cord lesions.13 A complete cord lesion was defined as complete paralysis and sensory loss below the spinal cord lesion.
Only 6 cases of primary parenchymal cavitation presenting within 3 months after spinal injuries classified as American Spinal Injury Association (ASIA) grade A-C (Table 1) have been reported.10,14,15 Posttraumatic syringomyelia presenting within 3 months has not been reported with an ASIA grade D or E spinal cord injury. The development of primary parenchymal cavitation represents a late complication most often developing many years after the spinal cord injury.

Scoliosis
Scoliosis is defined as an abnormal alignment or curve of the bony vertebral column with a lateral curvature of at least 10 degrees as measured by the Cobb angle on a standing upright x-ray of the spine (Figure 2). The Cobb angle is determined by an angle of curvature between the top and bottom of the lateral curve. A line is drawn parallel to the upper border of the upper vertebral body and another parallel to the lower border of the lowest vertebral body of the structural curve. Perpendiculars from these lines are drawn to cross each other and the angle between these perpendiculars is the angle of curvature. Adolescent idiopathic scoliosis is common with an overall prevalence of 0.47% to 5.2 %.16

Several studies have demonstrated scoliosis is much more common in those with syringomyelia than in the general population.17 The prevalence of scoliosis in people with CM and syringomyelia approaches 80% in some studies.11,18-21 Scoliosis is frequently associated with CM-I, occurring in up to 20% of cases, and even more frequently with CM-I in the setting of syringomyelia, in which rates are as high as 60%.22 It has been reported that 64% of children with a syrinx initially presented for evaluation of scoliosis.23 In a meta-analysis of 51 prospective and retrospective studies of 8,622 cases of idiopathic scoliosis, isolated syringes were identified in 3.4%, isolated CM in 3.0%, and CM with a syrinx in 2.5%.24 A study of 3,372 children with scoliosis showed that 8.3% had CM and 8.4% had syringomyelia.25
Tethered Cord Syndrome
Typically, the spinal cord hangs loose in the spinal canal, free to move up and down with growth as well as with bending and stretching. The base of the spinal cord is at the L1-2 level. A tethered cord, however, is attached at the end of or at some point in the spinal canal (Figure 3). In children, a tethered cord can force the spinal cord to stretch as they grow. In adults with a tethered cord, stretching during normal activity often leads to progressive spinal cord damage. In a study of 2,987 people with CM-I, 14% had tethered spinal cord syndrome.26 Lesions tethering the spinal cord included a lipomyelomeningocele, intraspinal lipomas, and a thick fatty filum terminale.

The clinical presentation of a tethered cord syndrome is quite variable with a combination of subcutaneous, neuro-logic, orthopedic, and urologic symptoms.27 The most commonly encountered cutaneous manifestations include cutaneous lipoma, dermal sinus, atypical dimple, deviation of the gluteal crease, hamartoma, hemangioma, or a port wine stain in the sacral region. Neurologic symptoms vary depending on age at presentation (Table 2).26

In infants, a tethered cord syndrome may present with reduced spontaneous leg movement, abnormal reflexes, foot asymmetry, or atrophic changes in the leg. Toddlers often demonstrate a delayed or abnormal gait. In school-age children, adolescents, and young adults, the presentation is an asymmetric motor and sensory dysfunction with painless trophic foot ulcerations. In advanced cases, hyperreflexia, and pain in the back and legs worsen with flexion or physical activities. The orthopedic symptoms include clubfoot, asymmetric leg length, atrophy of lower leg muscles, up subluxation, and scoliosis. The most common urologic symptomatology includes frequent urinary tract infections, urinary incontinence, and fecal soiling.27
Traumatic Aggravation of CM-I
Isolated case reports have reported a temporal relationship between minor head and neck trauma and the conversion of asymptomatic to symptomatic CM-I. In a reported case, a person age 37 collapsed after getting out of bed in the morning to go to the bathroom and subsequently had tiredness, confusion, and unsteadiness. Evaluation in the emergency department included neurologic examination that had no findings other than a positive Romberg test. Head CT and brain MRI showed descent of the cerebellar tonsils to the level of the posterior arch of C1, compatible with long-standing CM-I. The authors who reported the case, postulated that the CM-I made the neural tissue at the craniocervical junction, especially the cerebellum, more susceptible to a concussion-type injury.28 A report of 2 individuals with recent history of minor head trauma suggested the presence of CM-I may have precipitated sudden death.29 In another report, a person age 35 who was involved in a motor vehicle accident and had a flexion injury to the neck characterized by pain developed worsening head and neck pain, numbness in the extremities, gait difficulty, and incoordination 6 months later. In this case, it was assumed a transient cervical spinal cord dysfunction was provoked by a flexion injury to the neck, superimposed on a preexistent asymptomatic CM-I.30 A person involved in 2 separate rear-end motor vehicle accidents presented with significant headache, neck pain, and left arm paraesthesia and on brain MRI had a CM-I, leading to referral for consideration of neurosurgical decompression of the foramen magnum.31
In a retrospective evaluation of 364 persons with symptomatic CM-I, trauma was the most commonly reported precipitating factor (24.5%).27 A retrospective hospital chart review identified 85 individuals who were hospitalized with symptomatic CM-I,32 12.9% of whom had a history of minor head or neck trauma preceding onset of symptoms. In 3.5% the onset of symptoms could be attributed to the trauma based on the inclusion criteria.
Understanding whether trauma aggravates CM-I requires an understanding of the difference between correlation and causation. Correlation is when 2 factors (or variables) are related, but 1 does not necessarily cause the other. Causation is when 1 factor (or variable) causes another. A person with a CM-I may be asymptomatic or symptomatic. The common symptoms of CM-I include headache, neck pain, dizziness, muscle weakness or numbness, insomnia, depression, and difficulty with gait and coordination. The most common symptoms of a cervical whiplash disorder are headaches and neck pain. The most common presenting symptoms in a patient with a concussion include headache and dizziness. Considering yearly prevalence of CM-I in the population as well as the yearly prevalence of concussions and cervical whiplash related to motor vehicle accidents, a certain degree of overlap exists. It should not be a surprise that people with CM-I will be involved in motor vehicle accidents and sustain concussions or whiplash. When a person with CM-I is involved in a motor vehicle accident, the symptoms of headache, neck pain, and dizziness may be caused by the concussion, a cervical sprain and strain (whiplash), or conversion of an asymptomatic to symptomatic CM-I. To evaluate which is causing symptoms, the following possibilities must be considered:
1. the CM-I is asymptomatic and the symptoms are related to the concussion and/or whiplash;
2. the CM-I was asymptomatic but became symptomatic due the motor vehicle accident, and the injured person had a concussion and/or whiplash;
3. the CM-I was asymptomatic but became symptomatic due the motor vehicle accident, and the injured person did not have a concussion and/or whiplash;
4. the CM-I was symptomatic, and the person did not have a concussion or whiplash or
5. the CM-I was symptomatic, and the person did have a concussion and/or whiplash.
A detailed history, physical examination, and follow-up observation may help to assess whether the individual had a concussion and/or whiplash. A systematic literature was conducted to try to determine whether a person with asymptomatic CM-I could become symptomatic as a result of head and neck trauma.33 Between 1984 and 2018, there were 10 case reports of people with a CM-I who became symptomatic after craniocervical trauma. In each case, the T2-weighted MRI obtained 24 to 48 hours after the traumatic event demonstrated a hyperintense signal abnormality in the spinal cord and brainstem, characterized by edema, cervicomedullary swelling, or cord contusion. These observations suggest that traumatic worsening of CM-I and syringomyelia requires MRI evidence of edema, swelling, or contusion within the spinal cord/brainstem region.
Summary
Several comorbid congenital anomalies occur with CM-I, the most common and serious of which are syringes, scoliosis, and tethered spinal cord syndrome. Syringes associated with CM-I are classified as noncommunicating syringomyelia whereas syringes associated with trauma are classified as primary parenchymal cavitations. The development of primary parenchymal cavitations most often occurs many years after spinal cord injury. Aggravation of CM-I is associated with MRI evidence of edema, swelling, or contusion within the spinal cord/brainstem region.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Headache & Pain
When Migraine Treatments Fails: Navigating Therapy Switching
Vincent Martin, MD, FACP, FAHS; Charles Turck, PharmD, BCPS, BCCCPVincent Martin, MD, FACP, FAHS; Charles Turck, PharmD, BCPS, BCCCP



