Special Report: Neuro-oncology
Neuro-oncology is a broad and rapidly evolving field comprising portions of neurology, neurologic surgery, pediatrics, medical oncology, radiation oncology, neuroradiology, neuropathology, cancer rehabilitation, and palliative care. Neuro-oncology covers diagnosis and management of primary and metastatic tumors of the central nervous system (CNS) and complications of systemic cancers or cancer treatments. The goal of this 2-part special report is to provide an overview of epidemiology, presentation, diagnostic evaluation, and management for the most common CNS tumors. Here, we focus on medical and surgical management of brain tumors.
High-Grade Glioma
High-grade gliomas (HGGs) are malignant rapidly progressive primary brain tumors. They are divided into anaplastic glioma (WHO grade III—anaplastic astrocytoma, anaplastic oligodendroglioma) and glioblastoma (WHO grade IV). Figure 1 provides a management algorithm for HGGs.
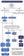
Surgical Treatment
Surgery is the primary initial treatment, and the goal is to resect as much tumor as possible without incurring permanent neurologic deficits, termed maximal safe resection. Resection of HGGs, which are infiltrative, requires removal of both tumor and infiltrated brain tissue. The extent of resection is an important prognostic factor and gross total resection (GTR) is associated with improved survival.1,2 When GTR is not feasible, subtotal resection (STR) should be performed for diagnosis and treatment of mass effect.3
Adjuvant Radiation Therapy
Glioblastoma Multiforme. Standard adjuvant therapy after maximal safe resection for glioblastoma multiforme (GBM) begins with radiotherapy (RT) (5 days/week) and temozolomide (TMZ) (7 days/week) for 6 weeks. This is followed by adjuvant TMZ on days 1 through 5 every 28 days for 6 cycles. This treatment improved median survival by 14.6 months compared with 12.1 months with RT alone.4
Isocitrate dehydrogenase type 1 or type 2 (IDH1/2) mutations and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation have no implications for upfront therapy. Methylation status of MGMT is associated with improved survival and predicts benefit of chemotherapy. In people with MGMT-methylated GBM, standard concurrent and adjuvant TMZ vs TMZ plus lomustine have similarly beneficial effects on survival, although a trend toward improved survival is seen with lomustine/TMZ.5
For people, more than age 70, treatment options are based on performance status as measured by the Karnofsky Performance Status (KPS) score. In those with good performance status (KPS > 60) and minimal comorbidities, treatment options include the Stupp protocol or a hypofractionated course of RT (2-3 weeks rather than standard 6 weeks) with concurrent and adjuvant TMZ. Hypofractionated RT in this age group is noninferior to standard 6-week RT course.6 In those with a KPS score less than 60, treatment options include hypofractionated RT alone, TMZ alone, or best supportive care. In this group, MGMT methylation status can inform the choice of RT or TMZ.7
A newer treatment option for GBM after RT is tumor treating fields (TTF), a locoregional therapy delivering low-intensity intermediate frequency (200 kHz) alternating electric fields to the tumor bed. It is hypothesized to arrest tumor cells in metaphase and inhibit further cell cycle progression. In intention-to treat-analysis, TMZ and TTF treatment resulted in a significantly longer progression-free survival (PFS, 6.7 vs 4 months, P < .001) and overall survival (OS, 20.9 vs 16 months, P < .001) compared with TMZ treatment alone.8 Given the survival benefit, use of the TTF device should be encouraged; it is safe and well tolerated but variably adopted.
Other therapies with limited roles include carmustine wafers and bevacizumab. Carmustine wafers implanted at the time of surgical resection are approved for treating both upfront and recurrent GBM. Carmustine wafer plus RT have shown survival benefit compared with RT alone,9 but no comparison has been made with with RT plus concurrent and adjuvant TMZ. Bevacizumab is an antiangiogenic agent (antivascular endothelial growth factor [VEGF] monoclonal antibody) that confers a benefit in PFS, but not OS and has more side effects than other treatments.10,11 Bevacizumab does have significant antiedema effects, which decreases steroid use and provides symptomatic relief. Bevacizumab is not routinely recommended for initial treatment.12
Anaplastic Gliomas. For anaplastic gliomas in individuals with KPS of 60 or more, combination therapy with RT combined with chemotherapy is recommended. Anaplastic oligodendroglioma with 1p/19q codeletion and KPS score 60 or more should be treated with fractionated RT plus procarbazine, lomustine, and vincristine (PCV) chemotherapy given before or after RT. This regimen has been shown to significantly increase median OS.13-15 Administering PCV after RT, rather than before, is better tolerated. Fractionated RT plus concurrent and adjuvant TMZ is also recommended and can be considered for individuals predicted to have difficulty tolerating PCV because of age or medical comorbidities. For anaplastic astrocytoma with KPS score 60 or more, fractionated RT followed by 12 cycles of adjuvant TMZ is recommended and shown to improve OS.16 For those with KPS score less than 60, treatment options are limited to single-modality therapies because of concerns about toxicity associated with combination regimens. Single-modality therapies include RT (hypofractionation is preferred over standard fractionation), TMZ alone (considered for tumors that are MGMT-promoter methylated), or palliative/best supportive care.
Surveillance and Recurrence
All HGGs eventually recur or progress. Surveillance should include brain MRI 2 to 6 weeks after RT, then every 2 to 4 months for 3 years, then every 3 to 6 months indefinitely.12 Early detection of recurrence is important for management but can be complicated by pseudoprogression. Pseudoprogression is a subacute treatment-related effect in which surveillance imaging may appear consistent with tumor progression (often in the first 3 months after RT) in the absence of true tumor progression. Pseudo-progression has reported frequency of 15% to 30% and is often asymptomatic, whereas true progression is often associated with clinical decline. Specialized imaging (eg, MR spectroscopy, MR perfusion, or positron emission tomography [PET]/CT) can occasionally help discern the process.
Management of recurrent HGGs depends on the extent of tumor and performance status. The efficacy of current treatments for recurrence is poor, and enrollment in clinical trials is preferable whenever possible. For individuals with recurrence and KPS score less than 60, consider best supportive care.
For those with KPS score of 60 or more, options include repeat resection for large well-circumscribed symptomatic tumor amenable to complete or near complete resection; a different chemotherapy regimen; or tumor-treating fields.
Chemotherapy options include retreatment with TMZ if there has been a long interval of time between stopping TMZ and tumor progression, particularly if the tumor is MGMT-promoter methylated. Bevacizumab is approved for the treatment of recurrence based on PFS improvement, although no improvement in OS has been observed. Other chemotherapy options include single-modality treatment with lomustine. Radiation often has a limited role for the treatment of recurrent HGG because of the short time interval between initial radiation treatment and recurrence; it can, however, be considered in out-of-field GBM recurrence.
Finally, TTF is also approved for treating recurrent GBM because it is proven safe; noninferiority to chemotherapy and effect on OS has not yet been determined.17
Low-Grade Glioma
Low-grade gliomas (LGGs) are diffusely infiltrative tumors divided into astrocytoma and oligodendroglioma (World Health Organization [WHO] grade II). Figure 2 provides an algorithm for the treatment of LGG.

Surgery
Surgery is an important diagnostic and therapeutic modality in patients with a presumed diagnosis of LGG on imaging. Important considerations include timing and extent of resection. Immediate surgery is warranted in those who present with a large mass or extensive neurologic symptoms. For those with small tumors and minimal symptoms, short-term observation and assessment for alternative diagnoses is often reasonable; however, because trends toward improved survival have been observed, early diagnostic surgery is preferred given a trend towards improved survival in observational studies.18,19
Maximal safe resection with a goal of GTR is recommended because, although limited and retrospective, available data show GTR is associated with delayed tumor progression and malignant transformation (ie, new gadolinium enhancement on follow-up imaging or higher-grade glioma on biopsy) as well as improved survival.20-22 Subtotal tumor resection, open biopsy, or stereotactic biopsy confer a higher risk for progression.
Adjuvant Therapy
Adjuvant therapy for LGGs depends on risk stratifications, the definitions of which vary in the literature. In general, a person is considered to have low risk if she or he is under age 40 and had GTR. Those over age 40, and/or who had STR are considered high risk.12 Other prognostic factors that may guide adjuvant treatment include tumor size, presence of neurologic deficits, and the tumor IDH mutation status.
People with LGG may be observed following surgery with close follow up to assess for potential progression. Those with high-risk LGG should be treated with adjuvant RT followed by sequential chemotherapy. The recommended regimen (level 1) is 6 cycles of PCV chemotherapy (category 1 recommendation), shown to result in significantly improved OS compared with RT alone (13.3 vs 7.8 years, P = .02) in people with newly diagnosed WHO grade II gliomas and at least 1 of 2 risk factors for disease progression (STR or age ≥ 40).23
Other adjuvant treatment options for high-risk LGGs include RT plus TMZ (concurrently or TMZ after RT) (level 2B recommendation. Because PCV is difficult to tolerate, it may be reasonable to use RT plus TMZ to treat individuals who are more than age 40 or have multiple comorbidities.
Recurrence
If possible resectable recurrence occurs, surgery is recommended to confirm recurrence and assess for malignant progression. If unresectable, obtaining a biopsy is critical to ensure accurate treatment because pathology drives further treatment options. After surgery or biopsy confirms recurrence, the treatment options are to consider a clinical trial, if eligible; try a different chemotherapy regimen; consider reirradiation, especially if PFS is more than 2 years after prior RT or if the new lesion is outside the prior RT target; and palliative/best supportive care in patients with KPS score less than 60.
Brain Metastases
Brain metastases are the most common intracranial tumors arising most commonly from primary tumors of the lung, kidney, breast, melanoma, and colon. Primary approaches to treatment include surgery, stereotactic radiosurgery (SRS), whole-brain radiation therapy (WBRT), and novel targeted therapies (eg, tyrosine kinase inhibitors and immunotherapy).
The choice of treatment depends on a person’s performance status, age, number of brain metastases, extent of extracranial disease, and histology of the primary tumor. Prognostic information is helpful and can be assessed with recursive partitioning analysis (RPA), graded prognostic assessment (GPA), or diagnosis-specific graded prognostic assessment (DS-GPA). The RPA is an index based on age, KPS score, control of primary tumor, and extracranial metastases and defines 3 classes of disease with median survival ranging from 2.3 to 7.1 months.24 The GPA is similar to RPA but incorporates the number of brain metastases because this has a significant prognostic impact.25 Within different GPA scores, median survival ranges from 3.4 months to 25.3 months. Because multiple GPA studies show that survival and prognostic factors vary widely by diagnosis, data from a retrospective database was used to design DS-GPA, which uses different factor for different diagnoses. Factors predictive of survival from lung cancer metastases are age, KPS score, number of brain metastases and extracranial metastases; from breast cancer are age, KPS score, and tumor subtype; and from melanoma or renal cell KPS score and number of brain metastases.26
Limited Brain Metastases
Surgery, Radiosurgery, and Adjuvent Therapy. For individuals with limited brain metastases, aggressive treatment should be considered, including surgery followed by adjuvant RT, RT alone, or systemic therapy in select individuals with small asymptomatic brain metastases. The choice between surgery plus SRS or SRS alone depends on the size, location and histology of metastases.
For a single brain metastasis, SRS has demonstrated efficacy and safety making both surgery plus postoperative SRS and SRS alone reasonable options. Treatment should be individualized because comparative data are lacking. Surgery plus postoperative SRS is preferred for a single large and symptomatic brain metastasis for relief from mass effect, lower morbidity, and higher local control. Because toxicity and local failure after SRS increase with the size of brain metastasis, SRS should be limited to lesions of 3 cm or less. Use of SRS alone in the initial management of limited (1-4) brain metastases less than 3 cm is also supported.27
Adjunctive WBRT for people with limited brain metastases who are eligible for SRS is no longer considered standard of care but may be considered on an individual basis. The benefit of improved intracranial disease control with adjunctive WBRT is offset by lack of OS benefit and worse cognitive outcomes.28-30
Chemotherapy. Systemic therapy (eg, chemotherapy) has rarely been used for brain metastasis because of poor intracranial efficacy and blood-brain-barrier penetration. Recent advances, especially targeted therapies in melanoma and nonsmall cell lung cancer (NSCLC), allow a more individualized approach to treatment. For individuals with small asymptomatic brain metastases who were not heavily pretreated, newer targeted treatment options may be available, including immunotherapy (Table 1).31-33

Multiple Brain Metastases
For peoplewith multiple brain metastases, the primary treatment is radiation therapy (WBRT or SRS). In those with a KPS score of 60 or more and low overall tumor volume (up to 10 tumors with a total cumulative volume ≤15 mL), SRS alone may be considered.32,34 For people with multiple large tumors or overall high tumor volume, WBRT remains the standard approach. The standard regimen for WBRT is 30 Gy in 10 fractions. First-line systemic therapy without RT (immunotherapy combination in melanoma and tyrosine kinase inhibitors [TKIs] in NSCLC) can still be considered for someone who is treatment naïve with multiple small asymptomatic brain metastases or who has a driver mutation.
Acute and delayed side effects associated with WBRT, most commonly include fatigue, alopecia and possible exacerbation of cerebral edema acutely. Steroid therapy should be given for 48 hours before WBRT in anyone with evidence of cerebral edema, whereas those with small metastases and no edema may not need steroids. Late side effects include neurocognitive decline, dementia, cerebrovascular disease, leukoencephalopathy, and radiation necrosis. Neuroprotective strategies to reduce risk of neurocognitive decline after WBRT are being studied and concurrent treatment with memantine and hippocampal-sparing radiation protocol shows some promise.35,36
Surveillance and Recurrence
After primary treatment, individuals should be followed with brain MRI every 2 to 3 months for 1 year, then as clinically indicated.12 If recurrence is found, treatment depends on prior intracranial therapies, systemic disease status and KPS score.
For individuals with systemic disease progression, KPS score less than 60, and limited systemic treatment, options include best supportive or palliative care or palliative reradiation. For those with stable systemic disease, options include surgery, reradiation, or systemic therapies. People previously treated with surgery who have local recurrence can be treated with revision surgery and/or SRS. For those who previously had WBRT, it should not be given again because of potential neurotoxicity; whereas for individuals who had SRS and have a distant metastatic recurrence, WBRT can be considered.
Meningioma
Treatment for individuals with meningioma consists of active surveillance, surgery, surgery plus RT, or RT alone (Figure 3). The choice of initial management depends on age, medical comorbidities, symptoms, size and location in relation to critical brain regions, and WHO grade of meningioma. Treatment plans should be discussed in a multidisciplinary panel to personalize available options appropriately, and individuals should be stratified by tumor size and presence of symptoms. For asymptomatic meningiomas, active surveillance is preferred for small tumors, with a general size cutoff of 3 cm or less.12

Small asymptomatic meningioma are usually discovered incidentally with neuroimaging, and most remain unchanged in size or show minimal change over years. A meta-analysis that included 2,130 people with incidental meningiomas showed that among the 51% who had active monitoring, approximately 8% developed symptoms and 25% needed intervention with a mean time to intervention of 25 months.37
Surgery
Symptomatic meningiomas or asymptomatic tumors that are more than 3 cm, expanding, or near a critical area (neurologic impairment is imminent) should be surgically resected. Complete surgical resection is the treatment of choice when feasible because it is associated with significantly improved 10-year PFS compared with partial/subtotal resection (75% vs 39%). The Simpson grading system, still in wide use by neurosurgeons, evaluates meningioma surgery based on the extent of tumor resection and dural attachment (grades I to V in decreasing degrees of completeness) and correlates with local recurrence rates. Overall tumor recurrence rates for grades I, II, III, and IV are 5%, 22%, 31%, and 35%, respectively.38
Adjuvant Treatment
Postoperative management depends on the WHO grade of meningioma, extent of resection, location of meningioma, and symptoms. Adjuvant RT is a standard part of initial therapy in all WHO grade III meningioma and WHO grade II with incomplete resection because it decreases recurrence rate. For WHO grade I meningioma with incomplete resection, adjuvant RT can be considered because it increases local control, although it does not increase OS. Adjuvant RT is typically reserved for cases that are considered difficult to resect. For WHO grade II meningioma with complete resection, the role of adjuvant RT is more controversial because there are no randomized data to guide treatment. Typically, the decision is informed by the criteria used to classify the meningioma as WHO grade II and the difficulty of the index surgical procedure. There are no Food and Drug Administration (FDA)-approved chemotherapy options for adjuvant treatment in meningioma.
Unresectable Meningioma
Radiation is considered standard of care for individuals who are not surgical candidates or who have an unresectable meningioma. Treatment with SRS has superior OS when compared with incomplete resection and similar 7-year PFS when compared with complete resection for meningiomas less than 35 mm.39 The meningioma grade determines the RT dose (Table 2).12

Surveillance and Recurrence
Most recurrences are local or adjacent to the prior tumor site. Surveillance brain MRI should be performed every 3 months for 1 year, then every 6 to 12 months for 5 years for low-grade meningiomas. Less frequent imaging is required beyond 5 years. Generally, malignant or recurrent meningiomas are followed more closely than grade I and II tumors.
For those who have recurrence after initial treatment, additional surgery and/or RT can sometimes be effective and occasionally permit long-term recurrence-free survival. The principles underlying the use of surgery or RT are similar to those for initial presentation, but appropriate management requires a consideration of the effects of prior surgery and/or RT.
Upon detection of recurrence, surgery is preferred whenever possible, followed by RT. For those who are not surgical candidates or who have an unresectable meningioma, consider RT. Observation is an option if treatment is not clinically indicated. Chemotherapy is reserved for individuals with an unresectable recurrence that is refractory to RT. Regimens include somatostatin analogs for somatostatin receptor-positive tumors, interferon α, sunitinib,41 bevacizumab, and bevacizumab with everolimus.40-42 These chemotherapy options are based on small phase 2 studies and therefore are a category 2B recommendation.12
Rehabilitation
Cancer rehabilitation is an interdisciplinary, critical area of medical care for people with neuro-oncologic tumors to address functional and neurologic deficits. Impairments may include cognitive dysfunction, muscle weakness, mobility difficulties, spasticity, sensory disruption, vision alterations, bowel/bladder disturbance, speech and swallow dysfunctions, among many others. Individuals have been reported to have 3 or more impairments occurring simultaneously that may arise because of neurologic tumor location, neurologic side effects of treatment, and/or coexisting comorbidities.43 Regardless of etiology, quality of life can be significantly affected. Rehabilitation improves function whether the tumor is primary or metastatic.44-51 Rehabilitation can occur in inpatient, outpatient, and home settings and involve a team that includes physiatrists, occupational therapists, physical therapists, speech therapists, and other disciplines in areas affecting care and care and quality of life (eg, oncology nurse navigators, nurses, pharmacists, chaplains, and behavioral therapists). Physiatrists specializing in neuro-oncologic rehabilitation diagnose the etiology of impairments, provide medicinal and procedural interventions, assess and prescribe durable medical equipment, and coordinate interdisciplinary rehabilitation. The primary goal of rehabilitation is function and quality of life; therefore, the patient is the central person on the team and the patient’s goals dictate rehabilitation interventions and priorities.
A person’s functional trajectory can fluctuate based on their tumor progression/recurrence and their medical treatments. The rehabilitation team must adjust goals and interventions appropriately while coordinating with the oncologic team.52 Although identifying and addressing functional changes early can help mitigate decline and potentially reduce hospital admissions, rehabilitation is underprescribed.53,54 Monitoring functional status is critical because integrated rehabilitation can help improve function, which affects quality of life and potentially timeliness to treatment.55,56
Palliative Care
Optimal supportive and palliative care is important in the management of anyone with primary or metastatic brain tumors. Effective supportive care results in symptomatic improvement and a better quality of life. Palliative care should be offered to anyone whose symptoms affect their quality of life. Hospice care should be discussed whenever the KPS score is less than 60.
Vasogenic Edema
Vasogenic edema usually surrounds brain tumors and contributes to significant morbidity. Systemic glucocorticoids are indicated in all persons with a brain tumor who have symptoms related to peritumoral edema. For those who are asymptomatic, steroids are not recommended because there are significant side effects from chronic steroid administration.
Dexamethasone is the preferred glucocorticoid because it has no mineralocorticoid activity.56 For severe symptoms, the initial dexamethasone regimen consists of a 10-mg loading dose followed by 4 mg given 4 times daily. For mild to moderate symptoms, a starting daily dose of 4 to 8 mg/day in divided doses is recommended. A response is usually seen within hours and maximal benefit is achieved within 1 to 3 days. After a response is seen, steroids should be tapered gradually (approximately every 4 days over a period of 2 weeks).57,58 For individuals who do not tolerate a steroid taper, a more protracted course may be required.
Glucocorticoids are effective but associated with many side effects (eg, insomnia, gastritis, peptic ulcer disease, steroid myopathy, and hyperglycemia), which can be reduced by using the lowest possible dose for the shortest possible duration. It should be noted that some retrospective studies suggest steroids use in individuals with GBM may be associated with decreased OS, independent of other confounding factors.59
Seizures
Seizures are a common symptom of brain tumors and can cause significant morbidity requiring aggressive management. Antiseizure medications are not recommended for seizure prophylaxis in individuals with brain tumors who have no prior history of seizures. Systematic review shows no difference between placebo and antiseizure medication for first seizure prevention.60 Individuals who have had a seizure related to brain tumor should be treated with antiseizure medication because of a high probability of recurrence. There are no trials to suggest the superiority of a particular antiseizure medication vs another. Generally a first line antiseizure medication is given as monotherapy at the lowest effective dose.
Conclusion
Neuro-oncology is a complex discipline requiring knowledge from various specialties and is ideally suited to multidisciplinary management strategies. Treatments include surgery, radiation therapy, and chemotherapy—including targeted therapies or immunotherapy. It is critical to achieve a balance between promoting survival and quality of life. Hospice or palliative care alone should be considered in any patient with poor clinical performance.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Imaging & Testing
Real-World Experience Using Biomarker Testing for the Evaluation of Acute Traumatic Brain Injury
Steve Rauchman, MDSteve Rauchman, MD



