Persistent Idiopathic Isolated Hypoglossal Nerve Palsy
Case 1
Clinical Presentation
A man, age 58, with a history of aspirin-exacerbated respiratory disease and sinus surgery approximately 30 years ago presented with a 6-year history of left-sided tongue weakness that had gradually increased to the point of causing dysarthria and dysphagia to solids and liquids. On physical examination, the tongue was positioned at the midline and symmetric in appearance at rest and with protrusion. Fasciculations were observed on the left hemitongue, and there was left-sided weakness. The rest of the neurologic and physical examinations were unremarkable.
Diagnostic Studies
Nerve conduction studies (NCS) and EMG confirmed left-sided hypoglossal nerve paresis. Imaging studies, which included head and neck MRI and MR angiography, showed extensive paranasal pansinusitis that did not appear to affect the course of the hypoglossal nerve. Nasal endoscopy revealed extensive postoperative changes from the prior sinus surgery. Bilateral middle meati obstruction was noted due to polyposis and polypoid mucosal edema, which was more severe on the right than on the left. Fiberoptic laryngoscopy results were unremarkable.
There were no findings from laboratory testing, which included a complete blood count, erythrocyte sedimentation rate, C-reactive protein, coagulation studies (prothrombin time [PT], international normalized ratio [INR], and activated partial thromboplastin time [APTT]), hematinics (ie, serum folate, red cell folate, and serum vitamin B12), comprehensive metabolic panel, immunology (ie, IgG, IgA, IgM, C3, C4, and serum electrophoresis), virology (ie, herpes simplex virus [HSV], cytomegalovirus [CMV], Epstein-Barr virus [EBV], and Lyme disease), autoimmune antibodies (ie, antineutrophil cytoplasmic antibodies, liver kidney microsomal antibodies, smooth muscle antibodies, and parietal cell antibodies), and cerebrospinal fluid (CSF) culture and analysis.
Follow-Up
At our most recent follow-up with this patient, his symptoms had been gradually progressing for 6 years.
Case 2
Clinical Presentation
A man, age 63, with no significant past medical history presented with a 2-year history of tongue weakness, leftward deviation at rest and on protrusion, and mild dysphagia and dysarthria. Although these impairments had been stable for 2 years, there was recent worsening after a viral upper respiratory illness. On physical examination, the left hemitongue appeared atrophic and contracted at rest with fasciculations. There was leftward deviation that increased with protrusion. The patient’s speech was dysarthric. His neurologic and other physical examinations were otherwise unremarkable.
Diagnostic Studies
Results of EMG and NCS revealed left hypoglossal nerve paresis. Neither MRI nor MR angiography showed relevant abnormalities at the initial symptom onset or after the worsening of symptoms 2 years later. The same laboratory tests were done as in Case 1 and there were no significant findings.
Follow-Up
At the patient’s most recent follow-up, symptoms had again been stable for 1 year since the viral infection, making the total duration of symptoms 3 years.
Case 3
Clinical Presentation
A man, age 30, with a past medical history of inflammatory bowel disease treated with mesalamine presented with atrophy and fibrillations on the left side of his tongue and a slight change in speech for 2 weeks. He was a body builder and a current 10-pack year cigarette smoker. Having researched his symptoms online, he was extremely worried about the possibility of amyotrophic lateral sclerosis (ALS). On physical examination, the left hemitongue was atrophied with fasciculations and leftward deviation on protrusion. The head and neck and neurologic examinations were otherwise normal.

Diagnostic Testing
Results of EMG, EEG, and NCS were normal. Initial imaging, which included contrast-enhanced CT, thin-cut MRI along the entire course of the affected hypoglossal nerve, and MR angiography showed no abnormalities. Creatine kinase was mildly elevated (206 mcg/L); all other laboratory results, which included those done in Case 1, were unremarkable.
Follow-Up
A repeat MRI taken 13 months later showed marked deviation and deformity of the left hypoglossal nerve root and neurovascular contact with a redundant left posterior inferior cerebellar artery (PICA) in the cisternal segment (Figures 1 and 2).

Discussion
Peripheral hypoglossal nerve lesions are associated with the physical examination findings seen in these 3 cases, including ipsilateral paresis, atrophy, fasciculations, and deviation on protrusion. Hypoglossal nerve palsy (HNP) is most commonly found after a head and neck surgery, trauma, or as an early clinical indication of underlying neoplasm or neurologic disorder.1 It usually manifests with other cranial nerve deficits. Isolated HNP (IHNP) is rare and because 50% of cases are idiopathic, IHNP is often a diagnostic conundrum.2 Most cases of idiopathic IHNP are transient, resolving in a matter of weeks like Bell’s palsy.3,4 Persistent idiopathic IHNP is very rare; a systematic literature search identified only 7 previously reported cases.5,6
Including the 2 idiopathic cases described here, all 9 cases of persistent idiopathic IHNP (4 women and 5 men) had a median age at onset of 42 years with a range of 17 to 76 years. In a review of 245 patients who presented with HNP, 15.1% were classified as idiopathic (1.2% were eventually diagnosed with ALS) but duration of symptoms and whether these were isolated was not addressed.1 Symptoms of INHP may be progressive as in Case 1 or exacerbated, as in Case 2 in which a previously existing cryptogenic condition apparently worsened with a superimposed infection.
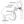

The patient in Case 3 was anxious about ALS to the point that his primary care physician prescribed benzodiazepines. The success of repeated imaging in detecting a likely cause of his symptoms demonstrates the utility of imaging for following up on patients with idiopathic IHNP. To our knowledge, this case is the first in which an etiology was identified after a provisional idiopathic diagnosis for such a long period of time. If a vascular etiology is confirmed, this patient may benefit from microvascular decompression of the hypoglossal nerve, which has reportedly been successfully performed.7
A 5-Segment Model of the Hypoglossal Nerve
In the established 5-segment model of the hypoglossal nerve (Figure 3), each segment tends to be affected by different conditions (Table 1).8-11 Other conditions that can affect any segment include Chiari malformation, Guillain-Barré syndrome,10 sarcoidosis, multiple sclerosis,12 and diabetes mellitus.13
The patients in the cases presented here all received the comprehensive diagnostic workup that has been proposed for evaluating hypoglossal nerve palsy (Table 2),5,14 which effectively excluded the above conditions. For the patient in Case 3, imaging findings specifically denoted a lack of abnormalities in the cisternal segment of the affected nerve. It is possible that a vascular disease process undetectable at the time of initial imaging progressed to become detectable on imaging 13 months later.
Neurologic examination cannot localize hypoglossal nerve lesions; imaging along the entire course of the nerve should be ordered. Contrast should be used for MRI and CT. For MRI, gadolinium enhanced T1W1 sequences are optimal for detecting most hypoglossal nerve pathologies. For CT, addition of contrast improves visualization of nearby vasculature and tumors. Although CT will not show the nerve itself, CT is particularly complementary to MRI in the skull base segment.15,16
Summary
Persistent idiopathic IHNP is a rare phenomenon seen in adult men and women of all ages. In the 5-segment model of the hypoglossal nerve, each segment is vulnerable to a relatively specific set of diseases. Case 3, in which an etiology was found, suggests that although transient idiopathic IHNP is comparable to Bell’s palsy, some persistent cases may be more akin to trigeminal neuralgia, which is thought to be caused, up to 90% of the time, by vascular compression.17-19 The numerous cadaveric dissections that demonstrated the frequency of this etiology for trigeminal neuralgia are unlikely to be feasible for persistent IHNP anytime soon due to its rarity.
1. Stino AM, Smith BE, Temkit M, Reddy SN. Hypoglossal nerve palsy: 245 cases. Muscle Nerve. 2016;54(6):1050-1054.
2. Khoo SG, Ullah I, Wallis F, Fenton JE. Isolated hypoglossal nerve palsy: a harbinger of malignancy. J Laryngol Otol. 2007;121(8):803-805.
3. Lee SS, Wang SJ, Fuh JL, Liu HC. Transient unilateral hypoglossal nerve palsy: a case report. Clin Neurol Neurosurg. 1994;96(2):148-151.
4. Combarros O, Alvarez de Arcaya A, Berciano J. Isolated unilateral hypoglossal nerve palsy: nine cases. J Neurol. 1998;245(2):98-100.
5. Sayan A, Abeysinghe AH, Brennan PA, Ilankovan V. Persistent idiopathic unilateral hypoglossal [corrected] nerve palsy: a case report. Br J Oral Maxillofac Surg. 2014;52(6):572-574.
6. Ilardi A, Moglia C, Cammarosano S, et al. Persistent idiopathic hypoglossal nerve palsy: a motor neuron disease-mimic syndrome? Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3-4):274-276.
7. Kuroi Y, Tani S, Ohbuchi H, Kasuya H. Microvascular decompression for hypoglossal nerve palsy secondary to vertebral artery compression: A case report and review of the literature. Surg Neurol Int. 2017;8:74.
8. Thompson EO, Smoker WR. Hypoglossal nerve palsy: a segmental approach. Radiographics. 1994;14(5):939-958.
9. Patron V, Roudaut PY, Lerat J, Vivent M, Bessede JP, Aubry K. Isolated hypoglossal palsy due to cervical osteophyte. EurAnn Otorhinolaryngol Head Neck Dis. 2012;129(1):44-46.
10. Keane JR. Twelfth-nerve palsy. Analysis of 100 cases. Arch Neurol. 1996;53(6):561-566.
11. Piccirilli M, Anichini G, Fabiani F, Rocchi G. Neurinoma of the hypoglossal nerve in the submandibular space: case report and review of the literature. Acta Neurochir (Wien). 2007;149(9):949-952; discussion 952.
12. Tritschler JL, Delouvrier JJ, Khoury M, Dehen H, Masson M. [Multiple sclerosis manifested by paralysis of the great hypoglossal nerve]. Nouv Presse Med. 1979;8(34):2756.
13. Semiz S, Fisenk F, Akcurin S, Bircan I. Temporary multiple cranial nerve palsies in a patient with type 1 diabetes mellitus. Diabetes Metab. 2002;28(5):413-416.
14. Freedman M, Jayasundara H, Stassen LF. Idiopathic isolated unilateral hypoglossal nerve palsy: a diagnosis of exclusion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):e22-26.
15. Alves P. Imaging the hypoglossal nerve. Eur J Radiol. 2010;74(2):368-377.
16. Learned KO, Thaler ER, O’Malley BW, Jr., Grady MS, Loevner LA. Hypoglossal nerve palsy missed and misinterpreted: the hidden skull base. J Comput Assist Tomogr. 2012;36(6):718-724.
17. Bowsher D. Trigeminal neuralgia: an anatomically oriented review. Clin Anat. 1997;10(6):409-415.
18. Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 2. Neurovascular compression of the trigeminal nerve in cadaveric controls and patients with trigeminal neuralgia: quantification and influence of method. Clin Anat. 1997;10(6):380-388.
19. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347-2360.
Disclosure
The authors have no relevant financial relationships to disclose.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Neuromuscular
Pediatric Neuromuscular Disorders Encountered in the Adult Neurology Clinic
Leslie Hayes, MDLeslie Hayes, MD - Neuromuscular
The Consequences of Delays in the Diagnosis of Generalized Myasthenia Gravis
John A. Morren, MD, FAAN, FAANEMJohn A. Morren, MD, FAAN, FAANEM



