Genetic Testing in the Care of Patients With Epilepsy
Historical Background
Epidemiologic research as well as analyses of individual families show that genetic factors play an important role in a broad spectrum of epilepsies. As early as 1951, Lennox analyzed data from more than 4,000 patients and reported an approximate incidence of epilepsy in 3.2% of family members of persons with essential (ie, nonlesional) epilepsy and an 84% concordance in monozygotic (ie, identical) twins versus a 10% concordance in dizygotic (ie, fraternal) twins with epilepsy.1 In 1995, it was discovered that a gene coding for the acetylcholine receptor α4 subunit is linked to autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),2 and this hallmarked a period of subsequent discoveries of individually relevant genes and variants causally associated with a range of epilepsies.
Today we know that genetic influences affect even patients with posttraumatic epilepsy in whom the emergence of epileptic seizures used to be attributed strictly to environmental factors.3 In complex disease, such as sporadic epilepsy, an estimated 5% to 10% of first-degree relatives manifest the disease compared to 50% in a fully penetrant autosomal dominant Mendelian disorder.4 There are increased options for and access to clinical genetic testing, and the expanding clinical relevance of molecular diagnosis and relative ease of access and direct-to-consumer marketing of DNA analysis may prompt physicians to initiate and then execute, the process of genetic testing in their clinical practice.
Understanding Genetically Defined Epilepsy Risk
Because most epilepsy seems to be sporadic, risk predictions based upon Mendelian inheritance are frequently not applicable. Understanding population-based risk for genetic epilepsies is often useful for patient counseling, particularly when discussing issues related to family planning or the merits of genetic testing. A 2014 epidemiologic study provides useful risk estimates that also validate some of the earlier observations.5 Among 2,439 first-degree relatives residing in a single county, the cumulative incidence of epilepsy to age 40 was 4.7% (standard error [SE], 0.60%). The risk for epilepsy was increased 3.3-fold (95% confidence interval [CI], 2.75-5.99) for first-degree relatives of persons with epilepsy, and this was similar among parents (4.5%; SE, 1.67%), siblings (4.8%; SE, 0.87%), and offspring (3.9%; SE, 0.89%). The risk was the greatest among relatives of probands with idiopathic epilepsies (standardized incidence ratio [SIR], 5.5), particularly among relatives of probands with idiopathic generalized epilepsies (SIR, 6.0) versus probands with idiopathic focal (SIR, 2.1) epilepsies. Relatives of probands with generalized epilepsy, had an 8-times higher risk for generalized epilepsy, but only a 2.5-times increased risk for focal epilepsy.5
Interpreting Genetic Epilepsy Variants
Genetic epilepsy can be a sole disease manifestation or a comorbidity within a syndrome with a multisystem involvement. Epilepsy is among the most common findings in chromosomal aberrations, identified in close to 40% of patients with a variety of genetic dysmorphic disorders.6 Together with developmental delay and intellectual disability, it is often dominant and highly disabling. The genetic lesions underlying human epilepsies range from aberrant chromosomal number or structure to highly devastating single-nucleotide substitutions. This widely varied molecular pathology warrants appropriate diagnostic platforms. Genetic testing has proven clinical relevance in confirming diagnosis, family counseling, prognostication, and, increasingly, in guiding therapeutic decisions. In a study of 611 consecutive newborns with seizures, 79 children were diagnosed with a broad spectrum of neonatal epilepsies. Although genetic diagnostic approaches varied, 58% of patients who were tested genetically received a molecular diagnosis.7 A similarly high diagnostic yield of genetic investigations is also found in early-life epilepsies.8 Clinically relevant genetic findings were found in 7.9%, 29.2%, and 27.8% of investigations using comparative genomic hybridization (CGH) microarrays, gene panels, and whole exome sequencing (WES), respectively. In this study of unselected patients with early-life epilepsies, genetic testing informed clinical diagnosis on par with imaging in 40% and 37.7% of patients, respectively.8 These results do not mean to suggest that genetic testing can replace core clinical dignostic modalities. Gene testing can be a powerful complementary diagnostic tool that ought to be considered early in the diagnostic course, in order to arrive at an accurate clinical diagnosis early and effectively. A helpful framework evaluating usefulness of genetic testing is available from the Center for Disease Control and Prevention.9 Commercial laboratories can submit information about their genetic testing platforms into a genetic testing registry supported by the National Institutes of Health.10
Types of Genetic Tests Available
Karyotype Testing and Comparative Genomic Hybridization
Although karyotype testing has been largely replaced by the more rapid and affordable evaluation via comparative genomic hybridization (CGH) microarray, it remains a method of choice in special conditions in which a detection by CGH may be falsely negative. An example is epilepsy due to a ringed chromosome 20, in which epilepsy severity and outcome correlate with the age of onset and the proportional presence of mosaicism of the ring chromosome.

The CGH microarray is used most commonly for detection of structural changes called copy-number variants (CNVs), which include deletions, duplications, or complex rearrangements of variable size that affect multiple genes. Large private CNVs spanning several megabases often result in a distinct genetic syndrome, such as Smith Magenis syndrome,11 in which epilepsy is one of several comorbidities. Large CNVs can be seen under a microscope using fluorescent in situ hybridization (FISH). However, the majority of CNVs are submicroscopic, recurrent, and occur at genomic hot spots. Among CNVs, those involving chromosomal regions 1q21.1, 15q11.2, 15q13.3, 16p13.11, 16p11.2, and 22q11.2 are associated with epilepsy, intellectual disability, and autism.4 About 3% of patients with generalized epilepsies of genetic etiology and 10% of persons with intellectual disability are carriers for CNVs involving the 15q11.2, 15q13.3, or 16p13.11 regions. An estimated 1.8% of persons with generalized epilepsies of genetic etiology have multiple CNVs. Testing for CNVs is an important component of a molecular investigation of epilepsy. Dravet’s syndrome, MECP2 duplication syndrome, Smith Magenic syndrome, and other syndromes can be caused by a single nucleotide variation, small insertions or deletions in a gene, or a CNV containing a critical gene.11,12 A review of 805 children referred for CGH to determine if CNVs were present found that the yield of CGH for diagnosis of epilepsy paralleled that seen in persons with autism. Pathogenic variants were identified in 40 children (5%) in the cohort.13 Another study of 143 adults with unexplained childhood-onset epilepsy and intellectual disability identified pathogenic or likely pathogenic CNVs in 23 cases (16.1%), and 5 patients tested had more than 1 CNV.14
The CGH microarray analysis is relatively inexpensive with a turnaround time of 2 to 4 weeks. As discussed here, published research supports the use of CGH to provide genetic diagnosis especially in individuals with epilepsy and intellectual disability and in patients with epilepsy and a family history of a neurodevelopmental disorder. As a complement to a single gene, gene panel, or exome sequencing in unresolved cases, CGH also has diagnostic value (Figure).
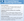
Diagnostic Sequencing, Gene Panels, and Whole Exome Analysis
Diagnostic sequencing typically involves evaluation using a single gene, gene panel, or whole exome analysis. The genetic and phenotypic heterogeneity of human epilepsies and cost considerations often prompt the use of a gene panel or WES. There are special circumstances when a focused single-gene analysis may be preferable (Box 1).
While the cost among commercial gene panels can be comparable, there are several important factors to consider when choosing which to use (Box 2). In a study of 33 patients with epilepsy, the yield of a 265-gene panel varied from 10% to 48.5%.17 In contrast, a review of the diagnostic yield of several commercial gene panels found that even a 38-gene panel may be sufficient to obtain genetic diagnosis in 93% of persons with epilepsy of genetic etiology.17,18 Some of the variables that affect the diagnostic utility of a panel are the relevance of genes on the panel, number of genes in pertinent biological pathways, age of epilepsy onset, the patient’s epilepsy type, and family history.17,19
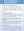
The WES analysis focuses on protein coding regions comprising approximately 1% to 2% of the genome, attributable to about 85% of disease-related mutations. This analysis may be a first line of investigation in epilepsies with features outside of well-known genetic epilepsy syndromes, or it may be an adjunctive test when there has been a negative result on single gene, gene panel, or CGH screenings. Sometimes, the decision to obtain WES is driven by practical reasons, such as approval by the insurance carriers. The reported diagnostic yield in unselected patient populations is in the range of 20% to 40%, although this will likely improve with ongoing discoveries and validation of epilepsy-related genes and variants.18 There are important caveats to consider when using WES for a molecular diagnosis of epilepsy. These caveats include that WES is not a discovery tool, gene and variant reporting is driven by the reported phenotype, and that WES generates an average of 20,000 variants, most of which are benign.20 Although the high number of variants increases the chances of finding pathogenic variants, it also increases the incidence of incidental findings that may affect patients and their immediate family and will necessitate appropriate counseling and further disposition (Figure).
Variant Interpretation
Variant interpretation follows the guidelines of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.21 Depending on the level of evidence (bioinformatics predictions, association with the disease phenotype, animal studies, and functional data), the variant is classified as either benign, likely benign, pathogenic, likely pathogenic, or as a variant of unclear significance (VUS). Testing laboratories deposit variants with available core phenotypic correlation and assertion criteria used in defining the variant class (benign, pathogenic, or VUS) into public databases, such as ClinVar.22 At the time this article was written, there were 432,354 unique variants of 30,180 genes in the ClinVar database. In collaboration with the Clinical Genome Resource these, termed ClinGen genes and variants, are being actively curated by expert groups with respect to relevance to a disease, variant pathogenicity, clinical actionability, and dosage sensitivity.23 These efforts are essential in addressing the extensive pool of variants with unclear clinical significance and in validating benign versus pathogenic variant status. While sophisticated bioinformatics tools can provide initial assessment of a variant functional effect, variant recurrence in association with an identical or similar epilepsy phenotype and its functional testing in model systems provides important levels of certainty. For example, a nonsynonymous variant N302S leads to a replacement of an evolutionary conserved amino acid asparagine (N) for serine (S) in a pore region of a sodium channel a subunit.24 A functional analysis showed that SCN1A gene-based N301S had no measurable sodium current, indicating a complete loss of function, whereas SCN3A gene-based N302S only slightly reduced channel activity.24
Counseling and Clinical Implications
Counseling
Genetic testing begins with genetic counseling that ideally happens before genetic testing is ordered (Figure). In the pretest counseling phase, it is critical to explain the purpose and form of the testing, associated cost, scientific terminology involved, expected results, effect on care, and possibility of incidental findings. It is also essential to learn about the expectations of the patient and family. The next counseling phase follows when results arrive. This posttesting session typically revolves around explanations of findings and implications for management, treatment, and health of other family members. It is helpful to refer patients to additional resources, such as the International League Against Epilepsy multilingual brochure on epilepsy genetics.25
Clinical Implications
Prognosis, Treatment, and Research. Results of large gene panels and WES include a large amount of genetic variation, and a candidate causal variant must make biological sense with the a priori clinical diagnosis. Pathogenic variants in several known epilepsy genes, such as KCNQ2, SCN1A, and SCN8A can be associated with relatively benign as well as severe epilepsy forms. Careful correlation of molecular diagnosis with the clinical profile and EEG findings can aid precise diagnosis and guide treatment, prognosis, family counseling, and referral to appropriate support groups.
Precision diagnostics creates opportunities for patients and families to organize around a clinical-genetic syndrome and has facilitated focused research with the ultimate goal of precision medicine. There is much interest for targeted compound development or repurposing of older drugs in a gene-specific matter. Although pilot compassionate drug trials for a patient or group of patients are important, rigorous unbiased trials are essential for safe and effective drug delivery. A recent randomized trial of oral quinidine in patients with epilepsy due to KCNT1 mutations is important in its concept and results. This single-center, inpatient, order-randomized, blinded, placebo-controlled, crossover trial of oral quinidine included 6 patients with severe ADNFLE due to KCNT1 mutation.26 The study showed an absence of efficacy on seizure control and a dose-limiting prolongation of the long QT interval even with serum quinidine levels well below the therapeutic range. Although small, this trial suggested use of quinidine in patients with KCNT1-related ADNFLE may not be effective and is coupled with considerable cardiac risks.26 This study is important as it paves the way to rigorous intervention trials in genetic epilepsies. The study also highlights the growing demand on precision molecular diagnostics that goes beyond variant identification and pathogenic classification but includes in-depth understanding of the variant functionality and drug response.
Negative Results. In many patients, genetic investigation will not yield actionable findings. This may be because of false negatives (ie, suboptimal analytical validity), a causal variant that is not yet known to be linked to epilepsy, failure to detect the causal variant, or a phenotype with multivariant causes including gonadal or somatic mosaicism. Although inherent detection failure is uncommon in gene panels, it is more common in WES.20 Human errors may account for some false negative results as evidenced by a recent review of SCN1A sequencing data.27 The variant quality or a location may not be amenable to detection by the applied testing platform. For example, WES will not detect pathogenic repeat expansions, methylation changes, or variants affecting noncoding regions. For this reason, negative test results may prompt use of other tests (eg, single-gene testing, methylation microarrays, or WES) aimed at detecting less common pathogenic variants. It is estimated that 10% of patients with Dravet’s syndrome with a presumed de novo SCN1A pathogenic variant result from a parental gonadal mosaicism in which a parent may only report febrile seizures or a very mild epilepsy.28 Patients with focal epilepsies caused by a focal neural migration defect may have variation present only in the dysplastic tissue and not in the lymphocytes used to collect DNA for testing.29

There are several ways to improve the clinical utility of genetic testing (Box 3). When results of genetic testing are negative, there are avenues for further action, including complementary genetic analyse or reevaluation, which is usually free of charge after 12 months. There are also options for referring patients with syndromic epilepsies to specific support groups because families are an essential driving force behind molecular research for such conditions. Family can opt to deposit the exome data into the Epilepsy Genetics Initiative (EGI), a program sponsored by Citizens United for Research in Epilepsy,30 apply to the Undiagnosed Disease Network,31 or be referred to the international consortia focused on epilepsy research.32
Conclusions
There is a growing understanding of genetic contributions to epilepsy etiology. Technologic advances and research progress have enabled an ever-growing clinical utility of molecular investigations of epilepsies. Genetic testing is improving the accuracy of clinical diagnostic of epilepsy, facilitating discoveries of comorbidities and their surveillance, and in some situations informing treatment decisions. While testing affords physicians and patients with improved understanding of the disease, they also face challenges related to potential stigma, incidental findings, negative or uncertain results, and all too often problems with access to precision diagnostics and care. Although federal and private funding, epidemiological, qualitative, clinical, and translational research have brought the field to the current state of clinical utility, ongoing multifaceted efforts are essential for improving patients’ access to genetic evaluations and precision care.
1. Lennox WG. The heredity of epilepsy as told by relatives and twins. JAMA. 1951;146:529-536.
2. Steinlein OK, Mulley JC, Propping P, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201-203.
3. Diamond ML, Ritter AC, Failla MD, et al. IL-1 beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia. 2015;56:991-1001.
4. Thomas RH, Berkovic SF. The hidden genetics of epilepsy—a clinically important new paradigm. Nat Rev Neurol. 2014;10:283-292.
5. Peljto AL, Barker-Cummings C, Vasoli VM, et al. Familial risk of epilepsy: a population-based study. Brain. 2014;137:795-805.
6. Alfei E, Raviglione F, Franceschetti S, et al. Seizures and EEG features in 74 patients with genetic-dysmorphic syndromes. Am J Med Genet A. 2014;164A:3154-3161.
7. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: characteristics of a prospective US cohort. Neurology. 2017;89:893-899.
8. Berg AT, Coryell J, Saneto RP, et al. Early-life epilepsies and the emerging role of genetic testing. JAMA Pediatr. 2017;171:863-871.
9. Centers for Disease Control. ACCE model list of 44 targeted questions aimed at a comprehensive review of genetic testing. Public Health Genomics website. https://www.cdc.gov/genomics/gtesting/ACCE/acce_proj.htm. Updated December 28, 2010. Accessed August 6, 2018.
10. Ottman R, Hirose S, Jain S, et al. Genetic testing in the epilepsies—report of the ILAE Genetics Commission. Epilepsia. 2010;51:655-670.
11. Goldman AM, Potocki L, Walz K, et al. Epilepsy and chromosomal rearrangements in Smith-Magenis Syndrome [del(17)(p11.2p11.2)]. J Child Neurol. 2006;21:93-98.
12. Wang JW, Kurahashi H, Ishii A, et al. Microchromosomal deletions involving SCN1A and adjacent genes in severe myoclonic epilepsy in infancy. Epilepsia. 2008;49:1528-1534.
13. Olson H, Shen Y, Avallone J, et al. Copy number variation plays an important role in clinical epilepsy. Ann Neurol. 2014;75:943-958.
14. Borlot F, Regan BM, Bassett AS, Stavropoulos DJ, Andrade DM. Prevalence of pathogenic copy number variation in adults with pediatric-onset epilepsy and intellectual disability. JAMA Neurol. 2017;74:1301-1311.
15. Hirose S, Scheffer IE, Marini C, et al. SCN1A testing for epilepsy: application in clinical practice. Epilepsia. 2013;54:946-952.
16. Chambers C, Jansen LA, Dhamija R. Review of commercially available epilepsy genetic panels. J Genet Couns. 2016;25:213-217.
17. Dunn P, Albury CL, Maksemous N, et al. Next generation sequencing methods for diagnosis of epilepsy syndromes. Front Genet. 2018;9:20.
18. Mercimek-Mahmutoglu S, Patel J, Cordeiro D, et al. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia 2015;56:707-716.
19. Myers KA, Johnstone DL, Dyment DA. Epilepsy genetics: current knowledge, applications, and future directions. Clin Genet. 2018:1-17.
20. Sands TT, Choi H. Genetic testing in pediatric epilepsy. Curr Neurol Neurosci Rep. 2017;17:45.
21. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424.
22. National Center for Biotechnology Information, US National Library of Medicine. ClinVar database website. https://www.ncbi.nlm.nih.gov/clinvar/. Published 2013. Updated 2018. Accessed August 6, 2018.
23. Clinical Genomic Resource website. https://clinicalgenome.org/about/. Published 2018. Accessed August 6, 2018.
24. Chen YJ, Shi YW, Xu HQ, et al. Electrophysiological differences between the same pore region mutation in SCN1A and SCN3A. Mol Neurobiol.2015;51:1263-1270.
25. Genetics commission of the International League Against Epilepsy. Epilepsy and genetics: things you want to know. https://www.ilae.org/files/dmfile/GeneticsPamphlet-20131.pdf. Published 2013. Accessed August 6, 2018.
26. Mullen SA, Carney PW, Roten A, et al. Precision therapy for epilepsy due to KCNT1 mutations: a randomized trial of oral quinidine. Neurology. 2018;90:e67-e72.
27. Djemie T, Weckhuysen S, von Spiczak S, et al. Pitfalls in genetic testing: the story of missed SCN1A mutations. Mol Genet Genomic Med. 2016;4:457-464.
28. de Lange IM, Koudijs MJ, van ‘t Slot R, et al. Mosaicism of de novo pathogenic SCN1A variants in epilepsy is a frequent phenomenon that correlates with variable phenotypes. Epilepsia. 2018;59:690-703.
29. Baek ST, Copeland B, Yun EJ, et al. An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat Med. 2015;21:1445-1454.
30. Citizens United for Research in Epilepsy website. https://www.cureepilepsy.org/. Accessed August 6, 2018.
31. Undiagnosed Diseases Network website. https://www.genome.gov/27550959/undiagnosed-diseases-network-udn/. Accessed August 6, 2018.
32. How to join Epi25. Epi25 Collaborative website. http://epi-25.org/joining-epi25/. Accessed August 6, 2018.
Disclosure
Dr. Goldman reports no financial or other relationships related to this article.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Epilepsy & Seizures
Pharmacology of Emerging Selective Sodium Channel Antagonists for the Treatment of Epilepsy
Raman Sankar, MD, PhD, FAAN, FAESRaman Sankar, MD, PhD, FAAN, FAES - Epilepsy & Seizures
Pharmacologic Management of Behavioral Challenges and Psychiatric Comorbidities in People With Epilepsy
Lauren Waldron, MDLauren Waldron, MD - Epilepsy & Seizures
Treatment of Developmental/Epileptic Encephalopathy With Spike-Wave Activation in Sleep
Lekha M. Rao, MD; Robert C. Stowe, MDLekha M. Rao, MD; Robert C. Stowe, MD



