Calcitonin Gene-Related Peptide, Monoclonal Antibodies, and Migraine
Migraine is a common primary headache disorder characterized by recurrent attacks of throbbing pain, associated symptoms (nausea, vomiting, photo and phonophobia), a heightened sensitivity to stimuli, and other neurological symptoms. Migraine affects about 11 to 12 percent of the American and global population, making it the third most common disease worldwide. Chronic migraine, defined as 15 or more headache days per month (of which eight are migraine), affects nearly one percent of the population.1-4 Migraine results in enormous impact on headache-related disability, quality of life, and medical resource utilization.5-7
Many treatments are available for migraine, but no drug has made a more significant impact on acute treatment than triptans. Triptans are a class of medications which bind and stimulate serotonin receptors and are highly efficacious in aborting a migraine attack.8 Tripans work by inhibiting the activity of the trigeminal nerve and its’ connections with the brain. Its role in constriction of cerebral blood vessels is controversial.9 Following triptans, another groundbreaking development in migraine treatment was the use onabotulinumtoxinA for chronic migraine prevention which significantly improved quality of life.10,11 Recently, momentum has shifted toward development of the monoclonal antibody (mAb) directed at calcitonin gene-related peptide (CGRP) and its’ receptor, for the treatment of migraine and cluster headache.
Ahead, we will offer and overview of mAbs and the research currently being conducted for the use of mAbs in headache disorders.
CGRP and Migraine
First described in the early 1980s, CGRP is a 37 amino acid peptide, which is ubiquitously found in the central and peripheral nervous system. It is produced in two forms, alpha-CGRP and beta-CGRP.12 Cells produce alpha-CGRP by an alternate processing of the RNA responsible for calcitonin, hence the name. Despite the similar origin, CGRP has a unique function compared to calcitonin, which is primarily involved in calcium and bone metabolism. CGRP has long been proposed to play a integral role in nociception and the modulation of pain pathways in headache disorders.13 CGRP is a potent cerebral vasodilator and key molecule in the mediation of pain transmission in the trigeminovascular system as well as the pain pathways between the brainstem, thalamus and cortex.13-15 Elevated CGRP levels have been measured in migraine patients compared to healthy controls and CGRP infusion can induce migraine attacks in the experimental setting.16,17 The motivation for developing drugs targeting CGRP and its’ receptor has been based on these observations.
There are two types of drugs that have been developed to inhibit the action of CGRP. Several small molecules, termed “gepants,” were developed to target the CGRP receptor for acute and later preventive migraine treatment. These small molecules can cross the blood-brain barrier. They are similar in mechanism and efficacy to the triptans in treating acute migraine without the adverse effect of coronary vasoconstriction. However, development of these drugs has been met with roadblocks to adverse side effects such as hepatotoxicity or inadequate bioavailability. New gepants are now under development.
Therapeutic mAbs have the unique advantage of high target specificity, which decreases adverse side effects from binding outside of the intended target. In 1986, muromonab, was the first monoclonal antibody approved by the FDA.18 Currently, there are over 30 therapeutic mAbs available in the US and Europe.19 Monoclonal antibodies have been developed for the treatment of cancer, inflammatory diseases, and transplant rejection. The use of mAbs in neurology has been limited to multiple sclerosis, thus many neurologists have limited exposure to and knowledge of mAbs.
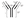
Figure 1 – Antibody Structure*
The Biology of An Antibody
Antibodies are globulin proteins, termed immunoglobulins (Ig), produced by B cells to target an antigen (a molecule capable of inducing an immune response). Antigens stimulate antibody production. There are five classes of antibodies: IgG, IgM, IgA, IgD, and IgE. IgG comprises 75 percent of serum immunoglobulins. Antibodies generally have a Y shape composed of two heavy chains and two light chains. The type of heavy chain determines the class of antibody.20 One end of the antibody is composed of both the heavy and light chain, is termed the fragment antigen-binding (Fab) arm. The end of the Fab arm contains the variable region of the antibody, which is responsible for selectivity to binding particular antigens. The Fab arm binds foreign protein at a site termed the epitope of the antigen. The specific region of the Fab that directly contacts the epitope is called the complementarity-determining region (CDR).21 The other end of the antibody is composed of only heavy chains and is termed the fragment crystallizable (Fc) arm. The Fc arm only has constant regions. The Fc arm binds the surface of host immune cells to trigger an immune reaction, known as effector function,22 which leads to antigen elimination.23 (See Figure 1)
Different clones of B cells produce different antibodies. Since each B cell produces its own unique antibody, this results in multiple different antibodies (polyclonal antibodies). The exception is B cell tumors, known as myelomas, which produce identical antibodies from clones of a single B cell (monoclonal antibodies). Monoclonal antibodies can be produced synthetically be the creation of hybridoma cells. These were first produced by immunizing mice with a particular antigen then culturing spleen cells from the immunized mouse with myeloma cells from another mouse. The cells fuse and reproduced indefinitely, resulting in the generation of large amounts of antibodies targeted at the intended antigen.20 Monoclonal antibodies derived from mice are called “murine” mAbs. Given that murine mAbs are completely foreign proteins, problems arise when used therapeutically in humans, including allergic reactions and the formation of anti-drug antibodies (ADAs). This propensity for a drug to generate ADAs is referred to as immunogenicity.21 Also problematic is that murine mAbs are metabolized quickly. This is due to weak binding of the antibody to the neonatal Fc receptor (FcRn), a molecule that protects IgG from being broken down.24
The next step to improve the safety and tolerability of the antibody was to develop more human antibodies. Scientists used genetic engineering to develop “chimeric” antibodies, meaning approximately 65 percent human-derived and the rest mouse-derived. In a chimeric mAb, the constant region, responsible for binding the human cell human-derived, and the variable region, responsible for binding the foreign antigen, is mouse-derived.25 Further developments lead to the creation of humanized mAbs in which only part (CDR region) of the variable region of the Fab arm is mouse-derived, the rest, approximately 90 percent, human-derived. Now technology has allowed for the development of 100 percent, or “fully human” mAbs, derived from genetically modified mice using antibody engineering. Fully human mAbs have the lowest potential for immunogenicity.26 The WHO developed a nomenclature system for naming mAbs, with the following suffixes: “-momab” for mouse mAbs, “-ximab” for chimeric mAbs, “-zumab” for humanized mAbs, and “-humab” for fully human mAbs (See Figure 1).27
The metabolism of mAbs is different that of small molecule drugs. The mAbs cannot be filtered by the kidneys or excreted in the urine due to their large molecular size. Instead mAbs are engulfed, or phagocytosed, by immune cells called macrophages and monocytes, which make up the reticuloendothelial system (RES). This is the primary mode of metabolism. Internal binding to the neonatal Fc receptor allows the mAb to be recycled to the cell surface. Monoclonal antibodies have to be given parenterally (IV, SQ, or IM). Since they have a half-life of about one month they can be administered much less frequently than small molecule drugs.

Monoclonal Antibodies for Migraine
Most migraine prevention treatments were originally developed for other indications, such as anti-epileptic drugs (AEDs), cardiac medications, anti-depressants, and onabotulinumtoxinA. The CGRP mAbs are the first drugs developed specifically for migraine prevention. Currently four CGRP monoclonal antibodies are being studied in phase 2 and 3 clinical trials. Three of these mAbs are directed at the ligand and one is directed at the CGRP receptor (See Table-1 comparison of the four CGRP mAbs).
Galcanezumab/LY2951742. Galcanezumab, or LY2951742, originally developed by Arteaus Therapeutics and subsequently acquired by Eli Lilly and Company, is a humanized CGRP mAb targeted at the ligand. It was studied in a phase 2, randomized, double-blind, placebo controlled study of patients with migraine four to 14 days per 28 days. Primary endpoint was mean change in mean headache days per month assessed at nine to 12 weeks. Galcanezumab was administered as subcutaneous injection once every two weeks for 12 weeks. The mean change in migraine days per 28 days was -4.2 or 63 percent, vs. -3 or 42 percent in placebo. Adverse events included injection site pain or erythema, upper respiratory tract infection, and abdominal pain, with no serious adverse events related to the study drug.28 Currently Eli Lilly is recruiting for two separate phase 3 studies for Galcanezumab, randomized, double-blind, placebo-controlled studies for episodic migraine (EVOLVE-2) and chronic migraine (REGAIN).
ALD403. ALD403,developed by Alder Biopharmaceuticals, is a humanized CGRP ligand mAb studied in a phase 2 randomized, double-blind, placebo-controlled trial for the prevention of frequent episodic migraine (five to 14 migraine days per 28-days). Patients were randomized to a single 1000 mg dose of intravenous ALD403 or placebo. The primary efficacy endpoint was change in frequency of migraine days from baseline to five to eight weeks, and was -5.6 days for ALD403 compared to -4.6 days in the placebo group. The most frequent adverse events were upper respiratory tract infection, urinary tract infection, fatigue, back pain, arthralgia, nausea and vomiting. There were no serious adverse events related to the study drug.29
ALD403 was also studied in a randomized, double-blind, placebo-controlled trial of ALD403, an anti-CGRP antibody in the prevention of chronic migraine (15 to 28 headache days per month, with at least eight days of which were migraine days). Patients were randomized to a single IV dose of 300 mg, 100 mg, 30 mg, 10 mg, or placebo. Primary endpoint was 75 percent responder rate for weeks 1 to 12, and was 33 percent in 300 mg group, 31 percent in 100 mg group, 28 percent in 30 mg group, 27 percent in 10 mg group and 21 percent in placebo group. The most common adverse event was upper respiratory tract infection, at 10 percent, followed by nasopharyngitis, nausea, sinusitis, dizziness, and bronchitis. No serious adverse events related to the study drug were reported. ALD403 is proceeding to phase 3 trials for chronic migraine (PROMISE 2) and frequent episodic migraine (PROMISE 1) as well as trials for SC and IM administrations of ALD403 every three months following phase 1 results warranting further study.30
TEV48125. TEV48125, owned by Teva Pharmaceuticals, originally developed by Labrys Biologics: Pfizer as LBR101, is a humanized CGRP ligand mAb. TEV48125 was studied for prevention of chronic migraine and high-frequency episodic migraine (eight to 14 migraine days per month) in two separate randomized, double-blind, placebo-controlled phase 2 trials. In the high frequency episodic migraine trial, patients were randomized to subcutaneous injections of TEV48125 225 mg, 625 mg, or placebo every 28 days, for three cycles in both studies. For the high-frequency episodic migraine study, the primary efficacy endpoint was change in migraine days in weeks nine to 12 compared to baseline. The change in migraine days was -6.3 for the 225 mg group vs. -6.1 in the 675 mg group and -3.5 in the placebo group. The secondary endpoint was change in headache days. The difference in change in headache days was -2.6 between the placebo group and the 225 mg group and -2.6 days between the placebo group and the 675 mg group.31
In the TEV48125 study for chronic migraine, patients were randomized to three 28-day cycles of one cycle of 675 mg of subcutaneous TEV48125 followed by two cycles of 225 mg, 900 mg for three cycles, or placebo. The primary efficacy endpoint was change from baseline in headache-hours during third treatment cycle. The mean change in headache hours at weeks 9 to 12 was -60 hours for the 675/225 mg group, -67.5 hours in the 900 mg group and -37.1 hours in the placebo group. The most common adverse reactions were mild injection-site pain and pruritus. No serious treatment related adverse events occurred.32 Patients are currently being recruited for two separate phase 3 TEV48125 trials for episodic and chronic migraine.
Erenumab/AMG334. Finally, Erenumab, or AMG334, co-developed by Amgen, Inc. and Novartis, is a fully human mAb to the CGRP receptor, in contrast to the other three CGRP mAbs, which are humanized and targeted to the ligand. Erenumab was studied in a randomized, double-blind, placebo-controlled phase 2 trial for episodic migraine (four to 14 migraine days per month) prevention. Patients were randomized to monthly subcutaneous AMG334 7 mg, 21 mg, 70 mg, or placebo. The primary endpoint was mean change in monthly migraine days at week 12 from baseline. The mean change in monthly migraine days at week 12 was -3.4 days with AMG334 70 mg versus -2.3 days with placebo. Differences in outcomes for the 7 mg and 21 mg doses compared to placebo was not significant. The most frequently adverse events were nasopharyngitis, fatigue, and headache. No serious treatment related adverse events occurred.33
Though unpublished, Novartis has presented results from a randomized, 12-week, double-blind, placebo-controlled study of AMG334 for chronic migraine. Patients were randomized to three cycles of monthly subcutaneous injections of AMG334 70 mg, 140 mg, or placebo. The primary endpoint, change in monthly migraine days, assessed at weeks 9 to 12, was -6.6 days in 70 mg and 140 mg group, compared to -4.2 days in the placebo group. Change in monthly headache hours, assessed as secondary endpoint was -64.8 hours for 70 mg, -74.5 hours for 140 mg, and -55.2 hours for placebo.34 A phase 3 trial is currently being conducted on AMG334 for the prevention of chronic migraine (ARISE trial).35
Conclusion
CGRP has long been implicated to play an important role in migraine. Based on decades of research, the successful development of CGRP mAbs for migraine marks a long-awaited breakthrough in migraine treatment. CGRP mAbs are the first agents developed specifically for migraine prevention and have demonstrated promising efficacy in clinical trials. Migraine is a debilitating and often stigmatizing condition, in which patients are frequently treated with medications reappropriated from other indications, such as anti-depressants. Thus, the availability of an agent designed specifically for migraine, may serve to provide a sense of legitimacy and hope to the millions of people living with migraine.
Although the CGRP mAbs have been therapeutically promising, several factors may serve as a barrier to use of these agents. Wide accessibility and inconvenience is of concern, as these agents will be expensive, non-oral, and perhaps requiring a headache specialist or a headache specialty center for in-office administration.
Despite these barriers, great potential for impact on the medical field remains. The large amount of publicity generated by the success of these agents may lead to a heightened awareness and accelerated development of preventive migraine therapies designed for specific biological targets. The potential spectrum of use for CGRP mAbs outside of migraine is yet to be seen. Now being studied also for treatment of cluster headache, a disease notorious for lack of efficacious treatment options, CGRP mAbs may be useful in other non-headache conditions. Continued success of these agents may motivate drug developers to pursue novel therapies for other challenging pain conditions such as fibromyalgia or intractable back pain. Certainly practitioners await the availability of these agents with great anticipation during what marks a very exciting time for migraine research and an innovative step toward improving the quality of life for our patients. n

Clinton Lauritsen, DO is a Neurologist and Headache Fellow at the Headache Center at Thomas Jefferson University in Philadelphia.

Stephen Silberstein, MD is Professor of Neurology and Director of the Headache Center at Thomas Jefferson University in Philadelphia.
1. Lipton, R.B., et al., Migraine prevalence, disease burden, and the need for preventive therapy. Neurology, 2007. 68(5): p. 343-9.
2. Stovner, L., et al., The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia, 2007. 27(3): p. 193-210.
3. Buse, D.C., et al., Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache, 2012. 52(10): p. 1456-70.
4. Steiner, T.J., L.J. Stovner, and G.L. Birbeck, Migraine: the seventh disabler. J Headache Pain, 2013. 14: p. 1.
5. Adams, A.M., et al., The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia, 2015. 35(7): p. 563-78.
6. D’Amico, D., et al., Disability and quality of life in headache: where we are now and where we are heading. Neurol Sci, 2013. 34 Suppl 1: p. S1-5.
7. Messali, A., et al., Direct and Indirect Costs of Chronic and Episodic Migraine in the United States: A Web-Based Survey. Headache, 2016. 56(2): p. 306-22.
8. Ferrari, M.D., et al., Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet, 2001. 358(9294): p. 1668-75.
9. Goadsby, P.J., The pharmacology of headache. Prog Neurobiol, 2000. 62(5): p. 509-25.
10. Dodick, D.W., et al., OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache, 2010. 50(6): p. 921-36.
11. Lipton, R.B., et al., OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: Pooled results from the PREEMPT randomized clinical trial program. Cephalalgia, 2016. 36(9): p. 899-908.
12. Amara, S.G., et al., Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature, 1982. 298(5871): p. 240-4.
13. Goadsby, P.J., L. Edvinsson, and R. Ekman, Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol, 1988. 23(2): p. 193-6.
14. Eftekhari, S., et al., Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience, 2010. 169(2): p. 683-96.
15. Eftekhari, S. and L. Edvinsson, Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci, 2011. 12: p. 112.
16. Cernuda-Morollon, E., et al., Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology, 2013. 81(14): p. 1191-6.
17. Hansen, J.M., et al., Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia, 2010. 30(10): p. 1179-86.
18. Liu, J.K., The history of monoclonal antibody development - Progress, remaining challenges and future innovations. Ann Med Surg (Lond), 2014. 3(4): p. 113-6.
19. Buss, N.A., et al., Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol, 2012. 12(5): p. 615-22.
20. Levinson, W., Review of medical microbiology and immunology. 12th ed. 2012, New York: McGraw-Hill Medical. ix, 710 p.
21. Silberstein, S., R. Lenz, and C. Xu, Therapeutic Monoclonal Antibodies: What Headache Specialists Need to Know. Headache, 2015. 55(8): p. 1171-82.
22. Hansel, T.T., et al., The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov, 2010. 9(4): p. 325-38.
23. Delves, P.J. and I.M. Roitt, Roitt’s essential immunology. 12th ed. Essentials. 2011, Chichester, West Sussex ; Hoboken, NJ: Wiley-Blackwell. xiii, 546 p.
24. Kuo, T.T. and V.G. Aveson, Neonatal Fc receptor and IgG-based therapeutics. MAbs, 2011. 3(5): p. 422-30.
25. Morrison, S.L., et al., Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A, 1984. 81(21): p. 6851-5.
26. Harding, F.A., et al., The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs, 2010. 2(3): p. 256-65.
27. Linker, R.A. and B.C. Kieseier, Innovative monoclonal antibody therapies in multiple sclerosis. Ther Adv Neurol Disord, 2008. 1(1): p. 43-52.
28. Dodick, D.W., et al., Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol, 2014. 13(9): p. 885-92.
29. Dodick, D.W., et al., Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol, 2014. 13(11): p. 1100-7.
30. Dodick, D.W., et al., Randomized, double-blind, placebo-controlled trial of ALD403, an anti-CGRP antibody in the prevention of chronic migraine [presentation]. American Headache Society 58th Annual Scientific Meeting. June 2016.
31. Bigal, M.E., et al., Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol, 2015. 14(11): p. 1081-90.
32. Bigal, M.E., et al., Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol, 2015. 14(11): p. 1091-100.
33. Sun, H., et al., Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol, 2016. 15(4): p. 382-90.
34. Tepper S et al. Phase II, R., Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Erenumab (AMG 334) in Chronic Migraine Prevention. Poster presented at: 5th European Headache and Migraine Trust Annual Congress; September 15-18, 2016; Glasgow, UK.
35. ClinicalTrials.gov. Study to Evaluate the Efficacy and Safety of AMG 334 Compared to Placebo in Migraine Prevention (ARISE). https://clinicaltrials.gov/ct2/show/NCT02483585.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Epilepsy & Seizures
Pitfalls in Management of Intracranial Hypotension
Rohit Kushwah, MD; Sarbesh Tiwari, DMRohit Kushwah, MD; Sarbesh Tiwari, DM - Headache & Pain
When Migraine Treatments Fails: Navigating Therapy Switching
Vincent Martin, MD, FACP, FAHS; Charles Turck, PharmD, BCPS, BCCCPVincent Martin, MD, FACP, FAHS; Charles Turck, PharmD, BCPS, BCCCP



